Arsenic contaminated groundwater - a problem in India and the UK
|
Dominik Weiss, Jay Bullen, Hany Heiba, Andreas Kafizas, Tony Cass, Ramon Vilar, Peter Childs, Stephen Skinner
Imperial College London [email protected] |
Pascal Salaun
University of Liverpool Priyanka Mondal, Swachchha Majumdar CSIR-Central Glass and Ceramic Research Institute |
ECG Bulletin January 2019
The identification of arsenic (As) as a potent carcinogen led the World Health Organisation (WHO) in 1993 to revise their guidelines for maximum As content in drinking water from 50 µg/L to 10 µg/L. Today, an estimated 120 million people throughout the world are exposed to unsafe levels of As in their drinking water.
|
Arsenic naturally occurs in shallow groundwater aquifers in many countries. India and Bangladesh are among the worst cases, where concentrations of up to 1000 µg/L have been reported (1). With respect to India, arsenic poisoning from drinking water is on the rise, with reports of new cases from the North Eastern states and the Ganga-Meghna-Brahmaputra plain in Bihar, from the states of Uttar Pradesh and Jharkhand in the Gangetic plain, and from the state of Assam in the Brahmaputra plain (2). In West Bengal, the worst affected Indian state, the estimated number of unsafe tube wells in eight of the most affected districts is 1.3 million, and the estimated population drinking arsenic contaminated water above 10 and 50 µg/L are 9.5 and 4.2 million, respectively. About 8% of the Indian population is under the threat of arsenic poisoning (3).
|
The challenge associated with contaminated groundwater, however, is not restricted to India. More than 85,000 private water supplies in the United Kingdom supply water to ~1% of households. In 2011, the Drinking Water Inspectorate reported that 3% of private wells studied contained arsenic at levels above present maximum contaminant level of 10 μg/L. Between 2011 and 2013, commissioned by the UK Health Protection Agency, the British Geological Survey undertook a sampling campaign of 512 properties in Cornwall served by private water supplies. Results from the study showed that five per cent of drinking water samples collected exceeded 10 μg/L arsenic concentration (4).
Arsenic treatment plants (ATP)
Access to drinking water in rural and remote areas away from an official water supplier requires tube wells. The ideal solution is to reach a non-contaminated deep aquifer, but that is often not economically feasible. Currently, there are about five million tube wells fitted with hand pumps in West Bengal, and more than 12 million tube wells in Bangladesh that provide drinking water from a depth of 15-60 m (1). Simple and small-scale systems to remove the arsenic at the point of collection are primarily based on fixed bed columns. However, difficulties with respect to operation and maintenance of these smaller units have emerged during field trials and frequent monitoring of arsenic levels would be required to guarantee safe operation. In addition, purification and sludge management is difficult and poses another form of hazard. Consequently, arsenic treatment plants with larger capacities are being developed.
Various ATP designs have been tested, including ion exchange, adsorption, ultrafiltration, reverse-osmosis, and adsorption-precipitation by metals (predominately ferric chloride) followed by coagulation (Figure 1). Chemical treatment and adsorption have emerged as the most effective solution, but fundamental challenges remain. This is reflected in a critical evaluation of the efficiency of ATP projects in removing arsenic from raw groundwater: a 2-year-long study, covering 18 ATPs from 11 manufacturers, installed in an arsenic affected area of West Bengal (5). Concentrations as high as 364 µg/L were found in the filtered water, and none of the ATPs could maintain arsenic in filtered water below the WHO provisional guideline value; only two could meet the standard value of 50 µg/L throughout. Furthermore, standard statistical techniques showed that ATPs from the same manufacturers were not equally efficient. Efficiency was evaluated based on point and interval estimates of the proportion of failure. The outcome was that many ATPs were identified as simply not fit for purpose.
The Indian government, NGOs, and international organisations have made great efforts to reduce arsenic exposure. The most successful program thus far has been the construction of deep tube wells and the testing of several millions of wells, which has led to a significant proportion of the affected population to switch from unsafe to safe wells. Unfortunately, with the high percentage of affected tube wells, the possibility of switching is limited. In some districts, depths of more than 180 m are required to reach brown sediments, and there is often a narrow depth range where arsenic concentrations are low while manganese concentrations and salinity are not too high (1).
In regions with limited options for alternative water sources, optimisation of water treatment remains urgent. The simplest method takes advantage of naturally present dissolved Fe(II), by co-precipitation of arsenic with hydrous ferric oxides that are formed within 15-60 min when pumped groundwater encounters air. Hydrous ferric oxides (with sorbed arsenic) are removed by settling or filtration through sand. However, studies have shown that the natural iron concentrations in many affected regions are too low to remove more than 50% of the arsenic.
Arsenic occurs in its inorganic form as oxyanions of the trivalent arsenite, As(III), or pentavalent arsenate, As(V), in the aquatic environment (6). The distribution between As(III) and As(V) in water depends critically on the redox environment and pH (6). Under typical anoxic groundwater conditions at near neutral pH of 7, As(III) is the predominant form of arsenic, which is far more toxic and mobile than As(V). Arsenite has low affinity to mineral surfaces, while arsenate adsorbs easily to solid surfaces, such as natural or synthetic metal oxides (7).
High concentrations of As(III) and high phosphate and silicate concentrations create added challenges for arsenic removal. As(III) sorbs weakly on iron oxides at circumneutral pH and is out-competed by the strongly sorbing phosphate and by weaker sorbing but abundant silicate and carbonate. Therefore, efficient arsenic removal requires oxidation of As(III) to the strongly sorbing As(V).
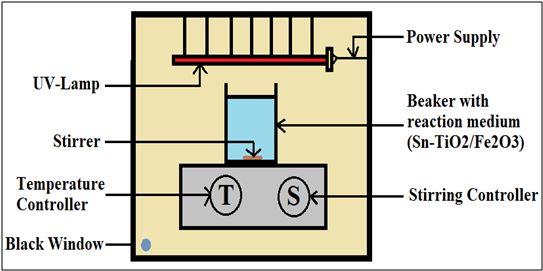
As(III) is typically oxidised to As(V) by chemical treatment, but the relatively high cost of oxidants and the formation of toxic by-products are significant drawbacks. Photocatalytic oxidation of As(III) is being investigated as an alternative to chemical oxidation (Figure 2). Photocatalytic As(III) oxidation with sunlight leads to significantly higher arsenic removal (40-90%) than precipitation alone (6).
Arsenic detection has always been challenging, especially on-site (8). On-line determination of arsenic in both the inflow and outflow streams in the ATP could provide several advantages. In the first instance, it would confirm whether arsenic levels in the outflow were below the maximum contaminant level and therefore confirm that the system was performing to specification. Secondly, it should be possible to then operate the treatment process dynamically, for example, by adjusting the flow rate or the adsorbent if it were approaching saturation.
Additionally it should also be possible to then predict when the removal system requires regeneration or replacement and identify early any unexpected failures. Field tests kits, mostly based on colorimetric methods, have been highly criticised for low reliability, time demand, and generation of toxic chemicals (Gutzeit method) (9, 10), despite major improvements (11). Other standard spectroscopic methods such as fluorescence or atomic absorption (hydride and graphite techniques) show excellent sensitivity. When coupled to chromatography, they are powerful tool for arsenic speciation, but these methods are expensive and laboratory-based, and thus not suited for rural on-site analysis.
Arsenic treatment plants (ATP)
Access to drinking water in rural and remote areas away from an official water supplier requires tube wells. The ideal solution is to reach a non-contaminated deep aquifer, but that is often not economically feasible. Currently, there are about five million tube wells fitted with hand pumps in West Bengal, and more than 12 million tube wells in Bangladesh that provide drinking water from a depth of 15-60 m (1). Simple and small-scale systems to remove the arsenic at the point of collection are primarily based on fixed bed columns. However, difficulties with respect to operation and maintenance of these smaller units have emerged during field trials and frequent monitoring of arsenic levels would be required to guarantee safe operation. In addition, purification and sludge management is difficult and poses another form of hazard. Consequently, arsenic treatment plants with larger capacities are being developed.
Various ATP designs have been tested, including ion exchange, adsorption, ultrafiltration, reverse-osmosis, and adsorption-precipitation by metals (predominately ferric chloride) followed by coagulation (Figure 1). Chemical treatment and adsorption have emerged as the most effective solution, but fundamental challenges remain. This is reflected in a critical evaluation of the efficiency of ATP projects in removing arsenic from raw groundwater: a 2-year-long study, covering 18 ATPs from 11 manufacturers, installed in an arsenic affected area of West Bengal (5). Concentrations as high as 364 µg/L were found in the filtered water, and none of the ATPs could maintain arsenic in filtered water below the WHO provisional guideline value; only two could meet the standard value of 50 µg/L throughout. Furthermore, standard statistical techniques showed that ATPs from the same manufacturers were not equally efficient. Efficiency was evaluated based on point and interval estimates of the proportion of failure. The outcome was that many ATPs were identified as simply not fit for purpose.
The Indian government, NGOs, and international organisations have made great efforts to reduce arsenic exposure. The most successful program thus far has been the construction of deep tube wells and the testing of several millions of wells, which has led to a significant proportion of the affected population to switch from unsafe to safe wells. Unfortunately, with the high percentage of affected tube wells, the possibility of switching is limited. In some districts, depths of more than 180 m are required to reach brown sediments, and there is often a narrow depth range where arsenic concentrations are low while manganese concentrations and salinity are not too high (1).
In regions with limited options for alternative water sources, optimisation of water treatment remains urgent. The simplest method takes advantage of naturally present dissolved Fe(II), by co-precipitation of arsenic with hydrous ferric oxides that are formed within 15-60 min when pumped groundwater encounters air. Hydrous ferric oxides (with sorbed arsenic) are removed by settling or filtration through sand. However, studies have shown that the natural iron concentrations in many affected regions are too low to remove more than 50% of the arsenic.
Arsenic occurs in its inorganic form as oxyanions of the trivalent arsenite, As(III), or pentavalent arsenate, As(V), in the aquatic environment (6). The distribution between As(III) and As(V) in water depends critically on the redox environment and pH (6). Under typical anoxic groundwater conditions at near neutral pH of 7, As(III) is the predominant form of arsenic, which is far more toxic and mobile than As(V). Arsenite has low affinity to mineral surfaces, while arsenate adsorbs easily to solid surfaces, such as natural or synthetic metal oxides (7).
High concentrations of As(III) and high phosphate and silicate concentrations create added challenges for arsenic removal. As(III) sorbs weakly on iron oxides at circumneutral pH and is out-competed by the strongly sorbing phosphate and by weaker sorbing but abundant silicate and carbonate. Therefore, efficient arsenic removal requires oxidation of As(III) to the strongly sorbing As(V).
As(III) is typically oxidised to As(V) by chemical treatment, but the relatively high cost of oxidants and the formation of toxic by-products are significant drawbacks. Photocatalytic oxidation of As(III) is being investigated as an alternative to chemical oxidation (Figure 2). Photocatalytic As(III) oxidation with sunlight leads to significantly higher arsenic removal (40-90%) than precipitation alone (6).
Arsenic detection has always been challenging, especially on-site (8). On-line determination of arsenic in both the inflow and outflow streams in the ATP could provide several advantages. In the first instance, it would confirm whether arsenic levels in the outflow were below the maximum contaminant level and therefore confirm that the system was performing to specification. Secondly, it should be possible to then operate the treatment process dynamically, for example, by adjusting the flow rate or the adsorbent if it were approaching saturation.
Additionally it should also be possible to then predict when the removal system requires regeneration or replacement and identify early any unexpected failures. Field tests kits, mostly based on colorimetric methods, have been highly criticised for low reliability, time demand, and generation of toxic chemicals (Gutzeit method) (9, 10), despite major improvements (11). Other standard spectroscopic methods such as fluorescence or atomic absorption (hydride and graphite techniques) show excellent sensitivity. When coupled to chromatography, they are powerful tool for arsenic speciation, but these methods are expensive and laboratory-based, and thus not suited for rural on-site analysis.
|
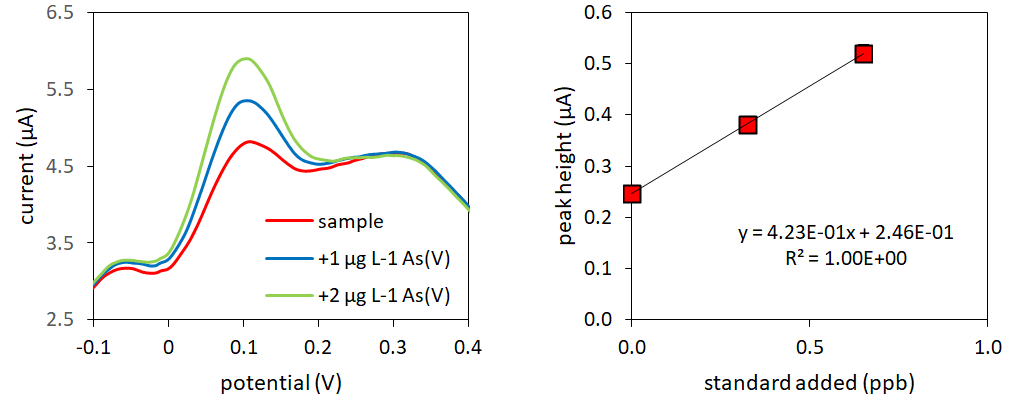
Voltammetric methods, which use cost effective, portable, low running cost electrochemical systems, are much more suited for rural on-site analysis (12) (Figure 3). Although there is an extensive literature on the electrochemical determination of arsenic, primarily using stripping voltammetry (8, 13, 14), all methods suffer from drawbacks: those developed on solid electrodes such as gold disc usually employ relatively high acidic conditions. More recent methods that use nanoparticles, self-assembled monolayers, biomolecules or synthetic polymers, have a limited/unproven long term stability and often require complex experimental procedures (e.g. for the preparation of the sensor surface). Nevertheless, stripping voltammetry remains at the forefront of on-site arsenic analysis, is formally approved, and is utilised by some companies for metal detections (8). Commercially available systems, however, tend to have high capital costs and may require technical skills to operate.
|
The development of a novel generation of ATP
A collaboration between Imperial College London, University of Liverpool and CSIR-Central Glass and Ceramic Research Institute
A collaboration between Imperial College London, University of Liverpool and CSIR-Central Glass and Ceramic Research Institute
Important progress in arsenic remediation has been made over the last years by Imperial College London, the University of Liverpool and CSIR-CGCRI. This research includes the development of mixed mineral oxides (TiO2/Fe2O3) which in combination with sunlight or a source of ultraviolet radiation photochemically oxidise arsenite to arsenate within minutes without the use of chemical oxidants and the production of toxic by-products (Figure 2) (15, 16) . The resulting As(V) species can then be easily and efficiently extracted by mineral oxides. Progress also includes the development of a low cost electrochemical sensors that accurately determine arsenic concentrations down to the lower ppb level. The aim of current work is to apply these two new techniques to an ATP developed by a team at the CSIR-CGCRI in Kolkata, led by Majumdar and co-workers. This system, patented in three countries (Chile, India and Taiwan) and widely used in rural areas in India, combines the use of an iron oxide sorbent with subsequent micro filtration. We propose to introduce the mixed mineral oxides instead of the iron oxides and to integrate the sensors at the inlet and outlet of the ATP.
Further Reading
The geology of groundwater arsenic contamination, arsenic speciation, toxicology, and methods of remediation have been the subject of many previous articles in the ECG Newsletter and ECG Bulletin. See the following issues: June 1998, January 1999, July 1999, February 2002, January 2003, July 2003, July 2004, January 2006, January 2009, September 2011. http://www.rsc.org/Membership/Networking/InterestGroups/Environmental/bulletin.asp
The geology of groundwater arsenic contamination, arsenic speciation, toxicology, and methods of remediation have been the subject of many previous articles in the ECG Newsletter and ECG Bulletin. See the following issues: June 1998, January 1999, July 1999, February 2002, January 2003, July 2003, July 2004, January 2006, January 2009, September 2011. http://www.rsc.org/Membership/Networking/InterestGroups/Environmental/bulletin.asp
References
- S. Hug, O. Leupin, M. Berg, Bangladesh and Vietnam: Different Groundwater Compositions Requeire Different Approaches to Arsenic Mitigation. Env. Sci. Technol. 42, 6318 - 6323 (2008).
- H. Chakraborti, Arsenic Groundwater Contamination in Middle Ganga Plain, Bihar, India: A Future Danger?”. Environ Health Persp 111, 1194-1201 (2003).
- D. Chakraborti et al., Status of groundwater arsenic contamination in the state of West Bengal, India: a 20-year study report. Molecular Nutrition & Food Research 53, 542-551 (2009).
- www.bgs.ac.uk/research/highlights/2013/arsenicSW.html
- M. A. Hossain et al., Ineffectiveness and poor reliability of arsenic removal plants in West Bengal, India. Environ. Sci. Technol. 39, 4300-4306 (2005).
- P. K. Dutta, S. O. Pehkonen, V. K. Sharma, A. K. Ray, Photocatalytic oxidation of arsenic(III): Evidence of hydroxyl radicals. Environmental Science & Technology 39, 1827-1834 (2005).
- K. Gupta, U. C. Ghosh, Arsenic removal using hydrous nanostructure iron (III)-titanium (IV) binary mixed oxide from aqueous solution. J. Hazard. Mat. 161, 884-892 (2009).
- S. Antonova, E. Zakharova, Inorganic arsenic speciation by electroanalysis. From laboratory to field conditions: A mini-review. Electrochemistry Communications 70, 33-38 (2016).
- C. M. Steinmaus, C. M. George, D. A. Kalman, A. H. Smith, Evaluation of two new arsenic field test kits capable of detecting arsenic water concentrations close to 10 mu g/L. Environmental Science & Technology 40, 3362-3366 (2006).
- D. Melamed, Monitoring arsenic in the environment: a review of science and technologies with the potential for field measurements. Anal. Chim. Acta 532, 1-13 (2005).
- J. Feldmann, P. Salaün, in Arsenic contamination of groundwater, S. Ahuja, Ed. (John Wiley & Sons, Oxford, 2008), chap. 8, pp. 179-205.
- M. M. Rahman et al., Effectiveness and reliability of arsenic field testing kits: Are the million dollar screening projects effective or not? Environmental Science & Technology 36, 5385-5394 (2002).
- J. Luong, E. Lam, K. B. Male, Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal. Methods, 6157-6169 (2014).
- Z. G. Liu, X. J. Huang, Voltammetric determination of inorganic arsenic. Trac-Trends in Analytical Chemistry 60, 25-35 (2014).
- M. D'Arcy et al., Adsorption of oxy-anions in the teaching laboratory: An experiment to study a fundamental environmental engineering problem. Journal of Chemical Education 91, 505-510 (2014).
- M. D'Arcy, D. Weiss, M. Bluck, R. Vilar, Adsorption kinetics, capacity and mechanism of arsenate and phosphate on a bifunctional TiO 2-Fe 2O 3 bi-composite. Journal of Colloid and Interface Science 364, 205-212 (2011).
- A. Cheng et al., Investigating Arsenic Contents in Surface and Drinking Water by Voltammetry and the Method of Standard Additions. Journal of Chemical Education 93, 1945-1950 (2016).