- Home
- About
- Environmental Briefs
-
Distinguished Guest Lectures
- 2023 Water, water, everywhere – is it still safe to drink? The pollution impact on water quality
- 2022 Disposable Attitude: Electronics in the Environment >
- 2019 Radioactive Waste Disposal >
- 2018 Biopollution: Antimicrobial resistance in the environment >
- 2017 Inside the Engine >
- 2016 Geoengineering >
- 2015 Nanomaterials >
- 2014 Plastic debris in the ocean >
- 2013 Rare earths and other scarce metals >
- 2012 Energy, waste and resources >
- 2011 The Nitrogen Cycle – in a fix?
- 2010 Technology and the use of coal
- 2009 The future of water >
- 2008 The Science of Carbon Trading >
- 2007 Environmental chemistry in the Polar Regions >
- 2006 The impact of climate change on air quality >
- 2005 DGL Metals in the environment: estimation, health impacts and toxicology
- 2004 Environmental Chemistry from Space
- Articles, reviews & updates
- Meetings
- Resources
- Index
The surprising polar troposphere
Anna Jones
British Antarctic Survey
ECG Bulletin July 2007
British Antarctic Survey
ECG Bulletin July 2007
Chemical processes in the troposphere are driven by a combination of factors. Emissions from the Earth’s surface, either natural or anthropogenic, are the primary source of trace gases in the troposphere. The energy driving many chemical reactions is derived from the Sun, hence the reactions are “photochemical”. Transport processes can distribute longer-lived trace gases to locations remote from their origin. In this article, Dr Anna Jones, from the British Antarctic Survey, describes some of the highly surprising phenomena that have been discovered in the polar troposphere over the past twenty years.
Introduction
An appropriate starting point is to consider what the polar regions are like, and to contrast the North Pole with the South Pole. Antarctica is a snow-covered continent, surrounded by the Southern Ocean and remote from major population centres. The overlying troposphere is therefore one of the cleanest parts of the Earth’s troposphere, and is perturbed only by very long-lived pollutants such as CO2 and methane. The Arctic contrasts somewhat, in that it comprises a polar ocean (ice-covered during the majority of the year) that is surrounded by continents (snow-covered to a greater or lesser extent). There are parts of the Arctic that are also remote and clean, but equally, there are areas that are exposed to pollutants, a well known example being Arctic Haze.
From this background, what was the polar troposphere expected to be like? The general a priori view was that the polar troposphere would be chemically fairly uninteresting, with low mixing ratios of the reactive trace gases like OH, HO2, NO and NO2. The snow was considered to be important because of its albedo effect (which indeed it is), but that otherwise it would merely act as a cap to emissions from land and ocean surfaces below.
An appropriate starting point is to consider what the polar regions are like, and to contrast the North Pole with the South Pole. Antarctica is a snow-covered continent, surrounded by the Southern Ocean and remote from major population centres. The overlying troposphere is therefore one of the cleanest parts of the Earth’s troposphere, and is perturbed only by very long-lived pollutants such as CO2 and methane. The Arctic contrasts somewhat, in that it comprises a polar ocean (ice-covered during the majority of the year) that is surrounded by continents (snow-covered to a greater or lesser extent). There are parts of the Arctic that are also remote and clean, but equally, there are areas that are exposed to pollutants, a well known example being Arctic Haze.
From this background, what was the polar troposphere expected to be like? The general a priori view was that the polar troposphere would be chemically fairly uninteresting, with low mixing ratios of the reactive trace gases like OH, HO2, NO and NO2. The snow was considered to be important because of its albedo effect (which indeed it is), but that otherwise it would merely act as a cap to emissions from land and ocean surfaces below.
Atmospheric chemists, who want to understand atmospheric composition and processes throughout the Earth’s atmosphere, were nonetheless motivated to study there. The polar troposphere is unusual for a number of reasons: it is very cold; during winter it remains dark for up to 24 hours per day, and during summer the reverse situation occurs with extended hours of daylight. It therefore provides a good testing ground for numerical models of the atmosphere. Furthermore, the polar troposphere (in particular over Antarctica) is one of few remaining parts of the atmosphere that are genuinely clean, providing an opportunity to study a natural background atmosphere. Finally, of course, the polar troposphere makes up a significant part of the atmosphere, so we really ought to know what it is like.
What was found was by no means what was expected. Here I will report on two of the real surprises held by the polar troposphere.
Tropospheric ozone depletion
Most people will have heard about atmospheric ozone in the context of the stratosphere. However, ozone is also present in the troposphere, in much smaller amounts, but nonetheless important; it is involved in production of OH which is the most reactive radical in the atmosphere, responsible in large part for the removal of long-lived (including pollutant) trace gases.
In the polar regions, it was assumed that surface ozone would have a simple seasonal cycle. A minimum in the summer was anticipated, resulting from destruction by photochemically-driven reactions; conversely, during winter, with photochemistry effectively switched off, ozone could be transported into the polar regions by the general movement of the atmosphere resulting in an annual maximum. And indeed, there are places, such as the South African base, Sanae, situated 170km inland from the coast of Antarctica, where such a seasonal cycle is observed. But it has turned out that there are significant deviations from this cycle observed elsewhere.
The first hint of something unusual came from measurements made at Alert, on the northern tip of Nunavut, Canada. Measurements were made during springtime, soon after the sun had risen from the winter darkness. Initially ozone concentrations were in line with what was expected, roughly 30 parts per billion by volume (ppbv). But then strange features were observed: ozone concentrations dropped suddenly to around instrumental detection limits of only a few ppbv; they remained low for a day or so, and then recovered to more normal amounts. These features were observed on several occasions during that first springtime study at Alert.
The first question then was – are these features real, or is there an artefact in the measurements? Further measurements made in the Arctic during subsequent springtimes revealed the same features, showing that this was a real atmospheric phenomenon.
What was found was by no means what was expected. Here I will report on two of the real surprises held by the polar troposphere.
Tropospheric ozone depletion
Most people will have heard about atmospheric ozone in the context of the stratosphere. However, ozone is also present in the troposphere, in much smaller amounts, but nonetheless important; it is involved in production of OH which is the most reactive radical in the atmosphere, responsible in large part for the removal of long-lived (including pollutant) trace gases.
In the polar regions, it was assumed that surface ozone would have a simple seasonal cycle. A minimum in the summer was anticipated, resulting from destruction by photochemically-driven reactions; conversely, during winter, with photochemistry effectively switched off, ozone could be transported into the polar regions by the general movement of the atmosphere resulting in an annual maximum. And indeed, there are places, such as the South African base, Sanae, situated 170km inland from the coast of Antarctica, where such a seasonal cycle is observed. But it has turned out that there are significant deviations from this cycle observed elsewhere.
The first hint of something unusual came from measurements made at Alert, on the northern tip of Nunavut, Canada. Measurements were made during springtime, soon after the sun had risen from the winter darkness. Initially ozone concentrations were in line with what was expected, roughly 30 parts per billion by volume (ppbv). But then strange features were observed: ozone concentrations dropped suddenly to around instrumental detection limits of only a few ppbv; they remained low for a day or so, and then recovered to more normal amounts. These features were observed on several occasions during that first springtime study at Alert.
The first question then was – are these features real, or is there an artefact in the measurements? Further measurements made in the Arctic during subsequent springtimes revealed the same features, showing that this was a real atmospheric phenomenon.
|
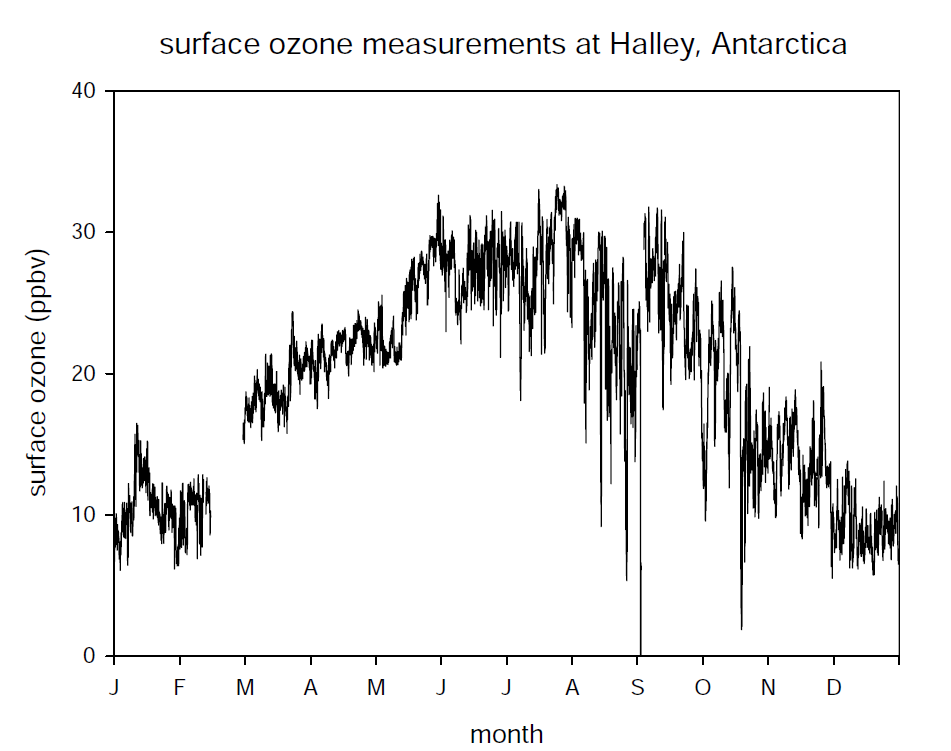
Given that these measurements were made in the Arctic, which can be subject to anthropogenic pollutants, the next question was – are these natural or man-made effects on ozone levels? The answer lay in Antarctic measurements, which showed that these features of rapid ozone loss, now known as Ozone Depletion Events (ODEs) were also measured at research stations in coastal Antarctica (see Figure 1). These data confirmed that ODEs were a natural phenomenon.
Ongoing research in both polar regions progressively built the now familiar picture of ODEs. They occur only during the spring; sometimes ozone concentrations drop from normal amounts to near-zero within the space of a few minutes; sometimes ozone loss can be less complete, and can occur more gradually; ozone can remain suppressed for several days; vertical profiling using balloons has shown that the ozone loss can extend up to ~1500 m in altitude and that they are contained within the planetary boundary layer by a capping inversion. An important observation was that ODEs are associated with transport of air masses over sea ice. |
So what causes ODEs? Early observations in the Arctic showed a very clear anti-correlation between surface ozone and what was referred to as “filterable bromide”, i.e. whatever was captured on a cellulose aerosol filter paper and gave a bromide peak when analysed on an ion chromatograph. Subsequent development both of theories and instrumentation, revealed a major role for gaseous bromine monoxide (BrO). A novel instrument, the Differential Optical Absorption Spectrometer, measured BrO and showed it to be unusually enhanced during ODEs. Measurements of BrO made on satellites, showed that during spring in the polar regions, BrO could be significantly enhanced over enormous areas of the sea ice zone. Again, the association with sea ice was clear
|
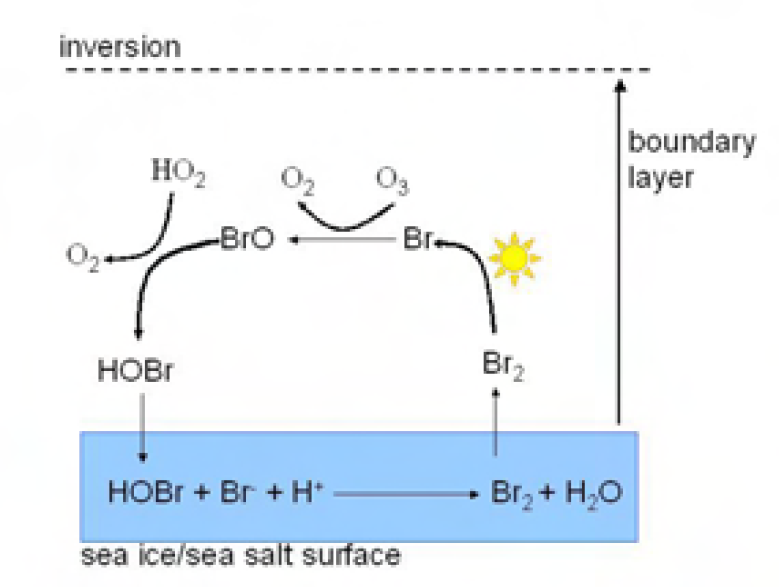
The mechanism that is driving surface ozone loss is known as the “Bromine Explosion”, and is autocatalytic in bromine in the sense that one gaseous bromine going into the system results in two gas-phase bromines coming out (Figure 2). Bromide is liberated from the quasi-liquid layer on some sort of sea ice surface (exactly which is still open to debate). The possible candidates include sea salt aerosol, sea salt deposited onto the snowpack, or frost flowers – delicate dendritic ice crystals which are highly saline and grow as new sea ice forms. Interestingly, elevated BrO has also been measured in other locations where large areas of exposed salt exist, such as salt pans and the Dead Sea.
ODEs have been significant for atmospheric chemists for a number of reasons. They highlighted new reaction pathways that are important for halogen chemistry in the boundary layer. They have a potential radiative role that could be relevant on a regional scale, if air that is low in ozone is mixed to higher altitudes where ozone is particularly radiatively important. Finally, they were completely unanticipated, and so provide a nice example that the natural world still holds surprises for us! |
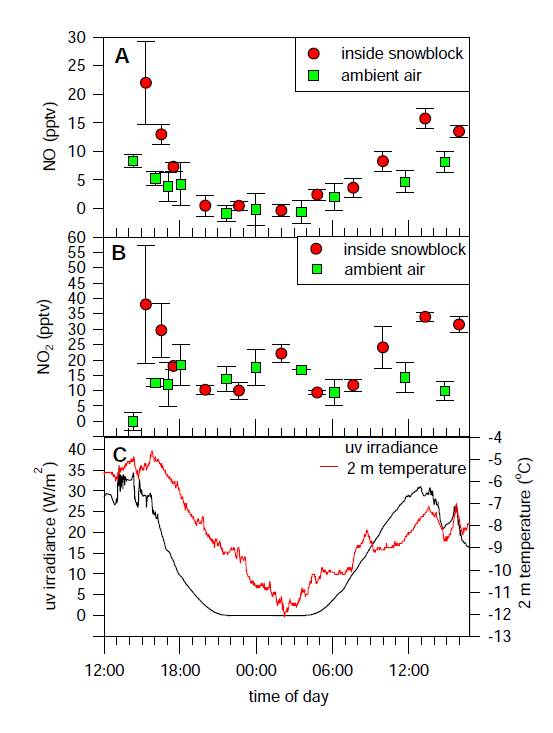
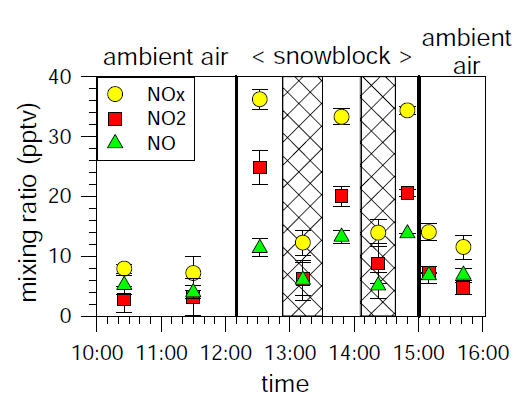
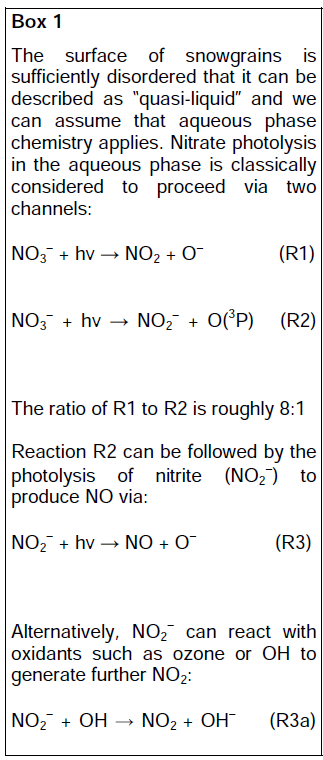
Normally this suite of reactions is associated with polluted regions, but mixing ratios of NO2 at the South Pole are sufficiently large for in situ production of ozone in the summertime boundary layer.
The production of NOx from snow was a case study to illustrate the mechanisms at work, the extent of the phenomenon, and the potential influence on other atmospheric chemistry. Other trace gases, such as HONO, HCHO, and ethene to name just a few, have also been shown to be produced by the action of sunlight on snow.
Summary
Snow photochemistry and the boundary layer ozone depletion phenomenon described earlier, are both new and exciting areas of science, and have opened a completely new way of thinking about the polar troposphere. Rather than being the chemically uninteresting place that many anticipated, it has turned out to be a fascinating region to study, and full of surprises!
Dr ANNA JONES
British Antarctic Survey,
Natural Environment Research Council,
High Cross,
Madingley Road,
Cambridge CB3 0ET
This article is based on a presentation by Dr Jones at the ECG’s 2007 Distinguished Guest Lecture and Symposium ‘Environmental Chemistry in the Polar Regions’.
Summary
Snow photochemistry and the boundary layer ozone depletion phenomenon described earlier, are both new and exciting areas of science, and have opened a completely new way of thinking about the polar troposphere. Rather than being the chemically uninteresting place that many anticipated, it has turned out to be a fascinating region to study, and full of surprises!
Dr ANNA JONES
British Antarctic Survey,
Natural Environment Research Council,
High Cross,
Madingley Road,
Cambridge CB3 0ET
This article is based on a presentation by Dr Jones at the ECG’s 2007 Distinguished Guest Lecture and Symposium ‘Environmental Chemistry in the Polar Regions’.