- Home
- About
- Environmental Briefs
-
Distinguished Guest Lectures
- 2023 Water, water, everywhere – is it still safe to drink? The pollution impact on water quality
- 2022 Disposable Attitude: Electronics in the Environment >
- 2019 Radioactive Waste Disposal >
- 2018 Biopollution: Antimicrobial resistance in the environment >
- 2017 Inside the Engine >
- 2016 Geoengineering >
- 2015 Nanomaterials >
- 2014 Plastic debris in the ocean >
- 2013 Rare earths and other scarce metals >
- 2012 Energy, waste and resources >
- 2011 The Nitrogen Cycle – in a fix?
- 2010 Technology and the use of coal
- 2009 The future of water >
- 2008 The Science of Carbon Trading >
- 2007 Environmental chemistry in the Polar Regions >
- 2006 The impact of climate change on air quality >
- 2005 DGL Metals in the environment: estimation, health impacts and toxicology
- 2004 Environmental Chemistry from Space
- Articles, reviews & updates
- Meetings
- Resources
- Index
Mind the gap: materials’ criticality – mitigation options and impacts
The theme of critical raw materials such as rare-earth elements has been of growing interest to industry and organisations. Concerns over criticality are likely to continue as a result of growing consumption and resource nationalism. The most appropriate mitigation option should be selected depending on the raw material and the application. The chemical sciences can provide solutions and enabling technologies.
|
Raw materials such as the rare earth elements, indium, platinum and gallium play a vital role in technologies such as flat panel displays, high-strength magnets, batteries, and catalytic converters. These materials are also employed in many “green” applications such as wind turbines, electric vehicles, solar panels, and low-energy lighting. There have been growing concerns over access to many of these raw materials, with the rare earths being the most recent high-profile example. These concerns have resulted in a number of studies to assess the criticality of raw materials. Although there is no universal definition of criticality, many studies combine an indicator of supply risk with another of importance (for example economic impact or importance to a technology) for a given group of materials (Figure 1).
These indicators themselves draw on factors such as geographical concentration of supply, end-of-life recycling rates, availability of substitutes, and uses in applications. Criticality is therefore a relative rather than an absolute measure, with assessments comparing materials against each other rather than against a defined baseline. |
Criticality is not closely tied to geological scarcity in the short term; most of these materials have large reserves, and it is other influences that have an impact on their supply.
Study methods are tailored to the needs of a particular interest group: Some studies take into account a broad range of metals, industrial minerals or, more recently, biotic materials; others assess a specifically chosen set of raw materials of particular interest to a particular market. Studies also rely on a specific context, such as a territory, a group of linked technologies, or materials critical to a business (1). Others include environmental issues within the assessment, either linked to supply risk or environmental impacts.
Despite these diverse interests and approaches, a broad consensus seems to have emerged as to which resources are critical. Analysis of 12 studies by Oakdene Hollins has identified 8 raw materials that are consistently identified in studies of criticality. They are beryllium, gallium, indium, magnesium, tin, tungsten, platinum group metals (PGMs), and rare earth elements. Although these materials present an easy-to-digest list, other materials should not be excluded from consideration, partly because criticality is context-dependent.
Responses
Six types of response can be identified to mitigate the criticality of materials. Different strategies are required depending on the particular material, circumstance, and application. Chemistry and the chemical sciences have a role to play in at least three of these: primary production, design and innovation, and resource efficiency.
Primary production. Improving and diversifying primary production has a key role to play in mitigating supply risk. Metals such as the PGMs, indium, and gallium also have some of the highest environmental impacts per tonne; improvements in primary production could help to reduce these impacts (2, 3). There is a clear role for chemistry in developing technologies to minimise processing losses, provide economic access to lower ore grades, reduce energy usage, and improve separation. In addition, many materials identified as critical are by-products of primary production but are often not separated because their economic value is small compared to that of their carrier metals (4). Developing new extraction and separation techniques for these metals would increase the attractiveness of by-production to metals producers.
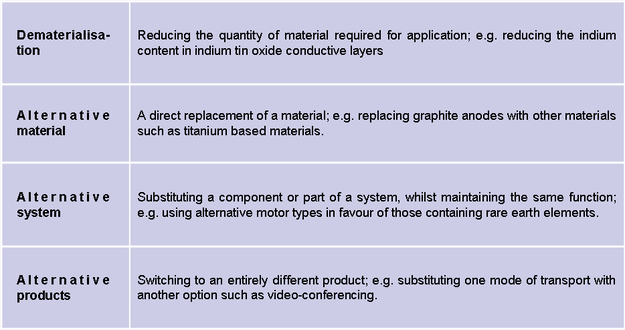
Design and innovation. Chemistry has a large role to play in providing technologies to substitute critical raw materials. Several different substitution strategies exist (Figure 2). These approaches are necessarily application-focussed, given that the complete substitution of material across all its uses is generally neither possible nor desirable. The Critical Raw Materials Innovation Network (CRM_Innonet), funded by the European Union’s Seventh Framework Program (FP7) and coordinated by the Chemistry Innocation Knowledge Transfer Network (CIKTN), aims to identify where priorities should lie (5).
Resource efficiency. Within this category sit options such as recycling, remanufacturing and reuse. Recycling rates of critical raw materials are believed to be very low (6), and where recycling activity does take place it does not necessarily reduce primary consumption.
Study methods are tailored to the needs of a particular interest group: Some studies take into account a broad range of metals, industrial minerals or, more recently, biotic materials; others assess a specifically chosen set of raw materials of particular interest to a particular market. Studies also rely on a specific context, such as a territory, a group of linked technologies, or materials critical to a business (1). Others include environmental issues within the assessment, either linked to supply risk or environmental impacts.
Despite these diverse interests and approaches, a broad consensus seems to have emerged as to which resources are critical. Analysis of 12 studies by Oakdene Hollins has identified 8 raw materials that are consistently identified in studies of criticality. They are beryllium, gallium, indium, magnesium, tin, tungsten, platinum group metals (PGMs), and rare earth elements. Although these materials present an easy-to-digest list, other materials should not be excluded from consideration, partly because criticality is context-dependent.
Responses
Six types of response can be identified to mitigate the criticality of materials. Different strategies are required depending on the particular material, circumstance, and application. Chemistry and the chemical sciences have a role to play in at least three of these: primary production, design and innovation, and resource efficiency.
Primary production. Improving and diversifying primary production has a key role to play in mitigating supply risk. Metals such as the PGMs, indium, and gallium also have some of the highest environmental impacts per tonne; improvements in primary production could help to reduce these impacts (2, 3). There is a clear role for chemistry in developing technologies to minimise processing losses, provide economic access to lower ore grades, reduce energy usage, and improve separation. In addition, many materials identified as critical are by-products of primary production but are often not separated because their economic value is small compared to that of their carrier metals (4). Developing new extraction and separation techniques for these metals would increase the attractiveness of by-production to metals producers.
Design and innovation. Chemistry has a large role to play in providing technologies to substitute critical raw materials. Several different substitution strategies exist (Figure 2). These approaches are necessarily application-focussed, given that the complete substitution of material across all its uses is generally neither possible nor desirable. The Critical Raw Materials Innovation Network (CRM_Innonet), funded by the European Union’s Seventh Framework Program (FP7) and coordinated by the Chemistry Innocation Knowledge Transfer Network (CIKTN), aims to identify where priorities should lie (5).
Resource efficiency. Within this category sit options such as recycling, remanufacturing and reuse. Recycling rates of critical raw materials are believed to be very low (6), and where recycling activity does take place it does not necessarily reduce primary consumption.
|
Not all applications lend themselves to recycling of critical raw materials, either because the materials are present in small quantities or are highly dispersed. For example, many metals are present in small quantities on printed circuit boards. Copper can be and is recovered; however many other metals are lost in the processing due to poor initial separation of components, shredding and then incorporation within other metal fractions or in the slag. There is an opportunity for chemists to develop technical and processing technologies to improve separation outcomes.
|
For example, research into reprocessing rare earth magnets is underway, which has long term possibilities for recovering these materials from wind turbines and electric vehicles. Other strategies such as product remanufacturing and reuse may be more practicable in many cases (7).
Outlook
Concerns over ‘criticality’ are likely to continue as consumption and resource nationalism grow. The most appropriate mitigation option will strongly depend on the raw material and the application. The chemical sciences can play a central role in addressing these issues. Raw materials criticality has also highlighted broader but related issues, including risks associated with the complete supply chain of products, traceability and provenance of materials, and the environmental impact associated with their production. The chemical sciences also have a role to play in addressing these concerns.
References
1. For examples, see EU DG ENTR (2010) – Critical raw materials for the EU, EU JRC (2011, 2013) – Critical Metals in Strategic Energy Technologies, and General Electric (2010) - Research Priorities for More Efficient Use of Critical Materials from a U.S. Corporate Perspective.
2. UNEP (2010), Priority Products and Materials: Assessing the Environmental Impacts of Consumption and Production, http://www.unep.org/resourcepanel/Portals/24102/PDFs/PriorityProductsAndMaterials_Report.pdf.
3. EU JRC Technical Reports (2012), Integration of resource efficiency and waste management criteria in European product policies – Second phase, see http://www.crni.ie/2013/02/integration-of-resource-efficiency-and-waste-management-criteria-in-european-product-policies-second-phase/
4. Joint study group report of the International Copper Study Group, the International Lead and Zinc Study Group, and the International Nickel Study Group (2012), Study and Directory of By-products of Copper, Lead, Zinc and Nickel.
5. For further informations see http://www.criticalrawmaterials.eu
6. UNEP International Resource Panel (2011), Recycling rates of metals, see http://www.unep.org/resourcepanel/Portals/24102/PDFs/Metals_Recycling_Rates_110412-1.pdf .
7. European Pathway to Zero Waste (EPOW) (2011) Study into the Feasibility of Protecting and Recovering Critical Raw Materials Through Infrastructure Development in the South East of England, see http://www.environment-agency.gov.uk/static/documents/Business/EPOW-recovering-critical-raw-materials-Summary-T5v2.pdf.
ADRIAN CHAPMAN
Oakdene Hollins
E-mail: [email protected]
This article is based on a presentation by Adrian Chapman at the ECG 2013 Distinguished Guest Lecture and Symposium held in the Science Room at Burlington House on Wednesday, March 20th 2013.
Outlook
Concerns over ‘criticality’ are likely to continue as consumption and resource nationalism grow. The most appropriate mitigation option will strongly depend on the raw material and the application. The chemical sciences can play a central role in addressing these issues. Raw materials criticality has also highlighted broader but related issues, including risks associated with the complete supply chain of products, traceability and provenance of materials, and the environmental impact associated with their production. The chemical sciences also have a role to play in addressing these concerns.
References
1. For examples, see EU DG ENTR (2010) – Critical raw materials for the EU, EU JRC (2011, 2013) – Critical Metals in Strategic Energy Technologies, and General Electric (2010) - Research Priorities for More Efficient Use of Critical Materials from a U.S. Corporate Perspective.
2. UNEP (2010), Priority Products and Materials: Assessing the Environmental Impacts of Consumption and Production, http://www.unep.org/resourcepanel/Portals/24102/PDFs/PriorityProductsAndMaterials_Report.pdf.
3. EU JRC Technical Reports (2012), Integration of resource efficiency and waste management criteria in European product policies – Second phase, see http://www.crni.ie/2013/02/integration-of-resource-efficiency-and-waste-management-criteria-in-european-product-policies-second-phase/
4. Joint study group report of the International Copper Study Group, the International Lead and Zinc Study Group, and the International Nickel Study Group (2012), Study and Directory of By-products of Copper, Lead, Zinc and Nickel.
5. For further informations see http://www.criticalrawmaterials.eu
6. UNEP International Resource Panel (2011), Recycling rates of metals, see http://www.unep.org/resourcepanel/Portals/24102/PDFs/Metals_Recycling_Rates_110412-1.pdf .
7. European Pathway to Zero Waste (EPOW) (2011) Study into the Feasibility of Protecting and Recovering Critical Raw Materials Through Infrastructure Development in the South East of England, see http://www.environment-agency.gov.uk/static/documents/Business/EPOW-recovering-critical-raw-materials-Summary-T5v2.pdf.
ADRIAN CHAPMAN
Oakdene Hollins
E-mail: [email protected]
This article is based on a presentation by Adrian Chapman at the ECG 2013 Distinguished Guest Lecture and Symposium held in the Science Room at Burlington House on Wednesday, March 20th 2013.
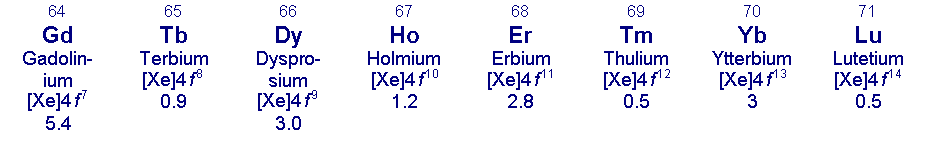
Lanthanum and the lanthanides (lanthanoids) - atomic number; symbol; element name; electronic configuration [M3+]; crustal abundance (ppm)
a S. R. Taylor, ‘Abundance of chemical elements in the continental crust: a new table’, Geochim. Cosmochim. Acta, 1964, 28, 1273. Some other values for comparison: Co 25 ppm; Mo 1.5 ppm; Cd 0.2 ppm.
b Radioactive; 14 radioisotopes reported.
Reading sources
Journal of Rare Earths, formerly Journal of the Chinese Rare Earth Society (English Edition). Published by Elsevier.
Handbook on the Physics and Chemistry of Rare Earths, North-Holland/Elsevier, Amsterdam, Vol. 1 (1978) to (current) Vol. 43 (2013).
T. Moeller, ‘The Lanthanides’, In Comprehensive Inorganic Chemistry, Pergamon Press, Oxford, 1973, Vol. 4, pp 1-101.
B. T. Kilbourn, A Lanthanide Lanthology: Part 1, A-L; Part 2, M-Z, Molycorp Inc., Fairfield, NJ, 1993.
S. A. Cotton, ‘Scandium, Yttrium & the Lanthanides: Inorganic & Coordination Chemistry’, In Encyclopedia of Inorganic Chemistry, 2nd edn., J. Wiley, Chichester, 2005, Vol. VIII, pp 4838-4877.
P. Atkins et al., Shriver & Atkins’ Inorganic Chemistry, 5th edn., Oxford University Press, Oxford, 2010, pp 579-592.
Journal of Rare Earths, formerly Journal of the Chinese Rare Earth Society (English Edition). Published by Elsevier.
Handbook on the Physics and Chemistry of Rare Earths, North-Holland/Elsevier, Amsterdam, Vol. 1 (1978) to (current) Vol. 43 (2013).
T. Moeller, ‘The Lanthanides’, In Comprehensive Inorganic Chemistry, Pergamon Press, Oxford, 1973, Vol. 4, pp 1-101.
B. T. Kilbourn, A Lanthanide Lanthology: Part 1, A-L; Part 2, M-Z, Molycorp Inc., Fairfield, NJ, 1993.
S. A. Cotton, ‘Scandium, Yttrium & the Lanthanides: Inorganic & Coordination Chemistry’, In Encyclopedia of Inorganic Chemistry, 2nd edn., J. Wiley, Chichester, 2005, Vol. VIII, pp 4838-4877.
P. Atkins et al., Shriver & Atkins’ Inorganic Chemistry, 5th edn., Oxford University Press, Oxford, 2010, pp 579-592.