- Home
- About
- Environmental Briefs
-
Distinguished Guest Lectures
- 2023 Water, water, everywhere – is it still safe to drink? The pollution impact on water quality
- 2022 Disposable Attitude: Electronics in the Environment >
- 2019 Radioactive Waste Disposal >
- 2018 Biopollution: Antimicrobial resistance in the environment >
- 2017 Inside the Engine >
- 2016 Geoengineering >
- 2015 Nanomaterials >
- 2014 Plastic debris in the ocean >
- 2013 Rare earths and other scarce metals >
- 2012 Energy, waste and resources >
- 2011 The Nitrogen Cycle – in a fix?
- 2010 Technology and the use of coal
- 2009 The future of water >
- 2008 The Science of Carbon Trading >
- 2007 Environmental chemistry in the Polar Regions >
- 2006 The impact of climate change on air quality >
- 2005 DGL Metals in the environment: estimation, health impacts and toxicology
- 2004 Environmental Chemistry from Space
- Articles, reviews & updates
- Meetings
- Resources
- Index
Overcoming nanoecotoxicological effects for sustainable remediation of nanomaterials
With a capacity for absorbing heavy metals plus its antimicrobial properties, graphene oxide is a promising material for water purification. The in vitro toxicity profile of graphene oxide precludes its direct use in water treatment, but the combination of graphene oxide (and related nanomaterials) with polymers retains the metal absorbent and antimicrobial properties, with the mitigation of possible toxic side effects.
The advancement of nanotechnology has led to the emergence of various types of metallic and carbon-based nanomaterials (1, 2). For example, graphene oxide (GO), a carbon-based nanomaterial, is a promising adsorbent for heavy metals (3-6). GO can adsorb significantly more heavy metals (Cu2+, Cd2+, Co2+) from aqueous solutions than unmodified carbon nanotubes and activated carbon (5, 7). Heavy metal sequestration by GO occurs mainly through oxygen-containing functional groups, such as carboxyl (COOH) and hydroxyl (OH). The removal of heavy metals is highly dependent on the pH because these functional groups can undergo protonation or deprotonation, depending on the pH (4, 8).
GO has anti-microbial properties (9, 10), which makes it a potentially useful material for water treatment. Concentrations as low as 45 mg/L to 85 mg/L lead to levels of microbial inactivation between 59% and 74%, while higher concentrations result in 100% microbial inactivation (10,11). Although GO has superior anti-microbial properties and better heavy metal removal capacity than most commonly used drinking water adsorbents, in vitro toxicity tests currently preclude the use of GO in water treatment. Several cytotoxicity studies with GO nanomaterials report problematic effects on human and other mammalian cells. As observed in antimicrobial studies, nanomaterial size, shape, dose, and exposure time were found to play an important role in their cytotoxic properties. In humans cell lines, GO can generate reactive oxygen species (ROS)(12) and, depending on the human cell line, these nanomaterials can be toxic at concentrations varying from 50 mg/L (13) to 85 mg/L (10). However, when these nanomaterials are combined with biologically compatible polymers (e.g. PEGylation), they exhibit negligible in vitro toxicity to many cell lines, even at high concentrations up to 100 mg/L (14, 15). Similar results were observed in animal models (13, 15). Furthermore, it was found that small sizes of GO-PEG in the range of 10 to 50 nm showed no obvious toxic side effects on treated mice in 40 days (16). In fact, it was observed that the nanomaterials were gradually excreted by mice without causing noticeable toxicity to the treated animals (17).
Overcoming toxicological effects
All previous studies showing toxicity of GO nanomaterials were carried out under pristine conditions. Recently, my research group determined the toxicity of a nanocomposite containing 30 mg/L of GO embedded in a biologically compatible polymer poly(N-vinyl)carbazole (PVK) at a concentration of 970 mg/L (18). In this study, we showed that this nanocomposite achieved 80% more inactivation of E. coli than pure GO by increasing the dispersibility of GO in aqueous solution (18). These results indicated that it is possible to reduce the concentration of GO to levels that are non-toxic to humans and animals and at the same time maintain the antimicrobial properties of GO.
In a further step, to determine the potential application of this nanocomposite for water treatment purposes, my research group coated commercially available membrane filters with poly(N-vinylcarbazole) (PVK), graphene (G), graphene oxide (GO), poly(N-vinylcarbazole)–graphene (PVK-G), and poly(N-vinylcarbazole)–graphene oxide (PVK-GO).
All previous studies showing toxicity of GO nanomaterials were carried out under pristine conditions. Recently, my research group determined the toxicity of a nanocomposite containing 30 mg/L of GO embedded in a biologically compatible polymer poly(N-vinyl)carbazole (PVK) at a concentration of 970 mg/L (18). In this study, we showed that this nanocomposite achieved 80% more inactivation of E. coli than pure GO by increasing the dispersibility of GO in aqueous solution (18). These results indicated that it is possible to reduce the concentration of GO to levels that are non-toxic to humans and animals and at the same time maintain the antimicrobial properties of GO.
In a further step, to determine the potential application of this nanocomposite for water treatment purposes, my research group coated commercially available membrane filters with poly(N-vinylcarbazole) (PVK), graphene (G), graphene oxide (GO), poly(N-vinylcarbazole)–graphene (PVK-G), and poly(N-vinylcarbazole)–graphene oxide (PVK-GO).
|
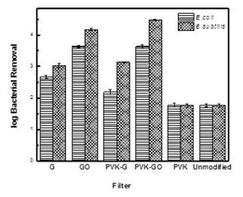
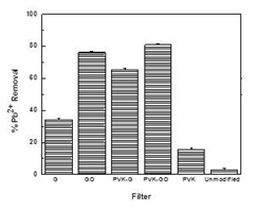
The results demonstrated that graphene-based nanocomposite membrane filters had improved antibacterial activity and heavy metal removal capacity compared with unmodified membrane filters, with the graphene-based nanocomposite membrane filters, PVK-GO–modified membrane filter exhibiting the best combination of antibacterial activity and heavy metal sequestration.
To estimate the effectiveness of the modified membrane filters to remove bacteria, bacterial plate counts were conducted in the filtrate after filtration of bacterial solutions. The results depicted in Figure 1 show that both GO and PVK-GO plate counts estimated 4 to 3 log removal for B. subtilis and E. coli, respectively. The amount of Pb2+ removed with the filter was also determined in the filtrate through atomic adsorption spectroscopy and reported as percentage Pb2+ removal (Figure 2). The results show that the PVK-GO modified membrane filter consistently exhibited the highest percentage Pb2+ removal, with 81.04%, followed by GO modified membrane filters, with 76.19% Pb2+ removal. The other modified membrane filters, such as PVK-G, G, and PVK, could remove 65.51%, 34.21% and 15.53%, respectively.
|
|
Conclusion
The dispersion of graphene–based nanomaterials in concentrations that are non-toxic to humans on the surface of commercially available membrane filters can significantly improve the antibacterial property and heavy metal removal capacity of commercial membrane filters. Therefore, it is possible to immobilise nanomaterials in nanocomposite forms for applications in water treatment.
The dispersion of graphene–based nanomaterials in concentrations that are non-toxic to humans on the surface of commercially available membrane filters can significantly improve the antibacterial property and heavy metal removal capacity of commercial membrane filters. Therefore, it is possible to immobilise nanomaterials in nanocomposite forms for applications in water treatment.
Acknowledgements
This project was supported by the National Science Foundation Career Award (NSF Award #104093). The author would also like to acknowledge Dr Yvonne Musico and Dr Catherine Santos.
This project was supported by the National Science Foundation Career Award (NSF Award #104093). The author would also like to acknowledge Dr Yvonne Musico and Dr Catherine Santos.
References
1. J. Warshaw, Dose–Response 10(3), 384 (2012).
2. K. Arivalagan, S. Ravichandran, K. Rangasamy, E. Karthikeyan, Int. J. ChemTech Res. 3(2), 534 (2011).
3. X. Wang, Y. Guo, L. Yang, J. Zhao, X. Cheng, J. Environ. Anal .Toxicol . 2(7) (2012).
4. Y. L. F. Musico, C. M. Santos, M. L. P. Dalida, D. F. Rodrigues, J. Mat. Chem . A 1(11), 3789 (2013).
5. G. Zhao, J. Li, X. Ren, C. Chen, X. Wang, Environ. Sci. Technol . 45(24), 10454 (2011).
6. G. Zhao et al., Dalton Trans. 40(41), 10945 (2011).
7. S. Wang, H. Sun, H. M. Ang, M. O. Tadé, Chem. Eng. J. 226, 336 (2013).
8. C. J. Madadrang et al., ACS Appl. Mater. Interfaces 4(3), 1186 (2012).
9. O. Akhavan, E. Ghaderi, ACS Nano 4(10), 5731 (2010).
10. W. B. Hu et al., ACS Nano 4(7), 4317 (2010).
11. Q. Bao, D. Zhang, P. Qi, J. Colloid Interface Sci. 360(2), 463 (2011).
12. Y. B. Zhang et al., ACS Nano 4(6), 3181 (2010).
13. K. Wang et al., Nanoscale Res. Lett. 6, 1 (2011).
14. Z. Liu, J. T. Robinson, X. M. Sun, H. J. Dai, J. Amer. Chem. Soc. 130(33), 10876 (2008).
15. X. M. Sun et al., Nano Res. 1(3), 203 (2008).
16. K. Yang et al., Nano Lett. 10(9), 3318 (2010).
17. K. Yang et al., ACS Nano 5(1), 516 (2011).
18. C. M. Santos et al., Chem. Commun. 47, 8892 (2011).
1. J. Warshaw, Dose–Response 10(3), 384 (2012).
2. K. Arivalagan, S. Ravichandran, K. Rangasamy, E. Karthikeyan, Int. J. ChemTech Res. 3(2), 534 (2011).
3. X. Wang, Y. Guo, L. Yang, J. Zhao, X. Cheng, J. Environ. Anal .Toxicol . 2(7) (2012).
4. Y. L. F. Musico, C. M. Santos, M. L. P. Dalida, D. F. Rodrigues, J. Mat. Chem . A 1(11), 3789 (2013).
5. G. Zhao, J. Li, X. Ren, C. Chen, X. Wang, Environ. Sci. Technol . 45(24), 10454 (2011).
6. G. Zhao et al., Dalton Trans. 40(41), 10945 (2011).
7. S. Wang, H. Sun, H. M. Ang, M. O. Tadé, Chem. Eng. J. 226, 336 (2013).
8. C. J. Madadrang et al., ACS Appl. Mater. Interfaces 4(3), 1186 (2012).
9. O. Akhavan, E. Ghaderi, ACS Nano 4(10), 5731 (2010).
10. W. B. Hu et al., ACS Nano 4(7), 4317 (2010).
11. Q. Bao, D. Zhang, P. Qi, J. Colloid Interface Sci. 360(2), 463 (2011).
12. Y. B. Zhang et al., ACS Nano 4(6), 3181 (2010).
13. K. Wang et al., Nanoscale Res. Lett. 6, 1 (2011).
14. Z. Liu, J. T. Robinson, X. M. Sun, H. J. Dai, J. Amer. Chem. Soc. 130(33), 10876 (2008).
15. X. M. Sun et al., Nano Res. 1(3), 203 (2008).
16. K. Yang et al., Nano Lett. 10(9), 3318 (2010).
17. K. Yang et al., ACS Nano 5(1), 516 (2011).
18. C. M. Santos et al., Chem. Commun. 47, 8892 (2011).