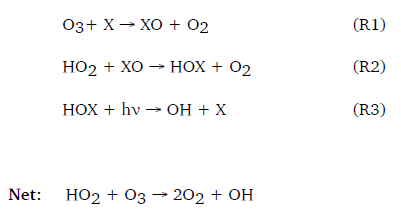An increasing role for iodine in the atmosphere
Tomás Sherwen
National Centre for Atmospheric Science and University of York
[email protected]
ECG Bulletin July 2019
National Centre for Atmospheric Science and University of York
[email protected]
ECG Bulletin July 2019
When people hear of halogens in the atmosphere, images of the ozone hole may come to mind. In the 1980s, destruction of ozone in the stratosphere – air over ten kilometres above the Earth – was all over the global news. The cause? Halogen-containing chlorofluorocarbons (CFCs). In recent decades, the ability of halogen species to destroy ozone in the region of air closest to the Earth, the troposphere, which we breathe day to day, has also received scientific attention.
Iodine in the troposphere
The chemistry of halogens in the troposphere differs from that in the stratosphere because of the lower energy of photons that make it down to the surface. In the stratosphere, the chemistry of chlorine dominates: here, the photon energy is high enough to break apart the strong bonds between chlorine and species such as carbon in CFCs. However, in the troposphere, the chemistry of more weakly bonded bromine and iodine species dominates instead. Reactions 1-3 (where X = Br or I) show the main ozone destroying cycle of bromine and iodine in the troposphere.
Iodine in the troposphere
The chemistry of halogens in the troposphere differs from that in the stratosphere because of the lower energy of photons that make it down to the surface. In the stratosphere, the chemistry of chlorine dominates: here, the photon energy is high enough to break apart the strong bonds between chlorine and species such as carbon in CFCs. However, in the troposphere, the chemistry of more weakly bonded bromine and iodine species dominates instead. Reactions 1-3 (where X = Br or I) show the main ozone destroying cycle of bromine and iodine in the troposphere.
The halogen monoxide radical forms by extracting an oxygen from ozone (O3), then reacting further with a peroxy radical (HO2) to form a hypohalous acid (HOX). The HOX rapidly breaks apart under light to regenerate the halogen radical, but not the ozone, thus destroying ozone.
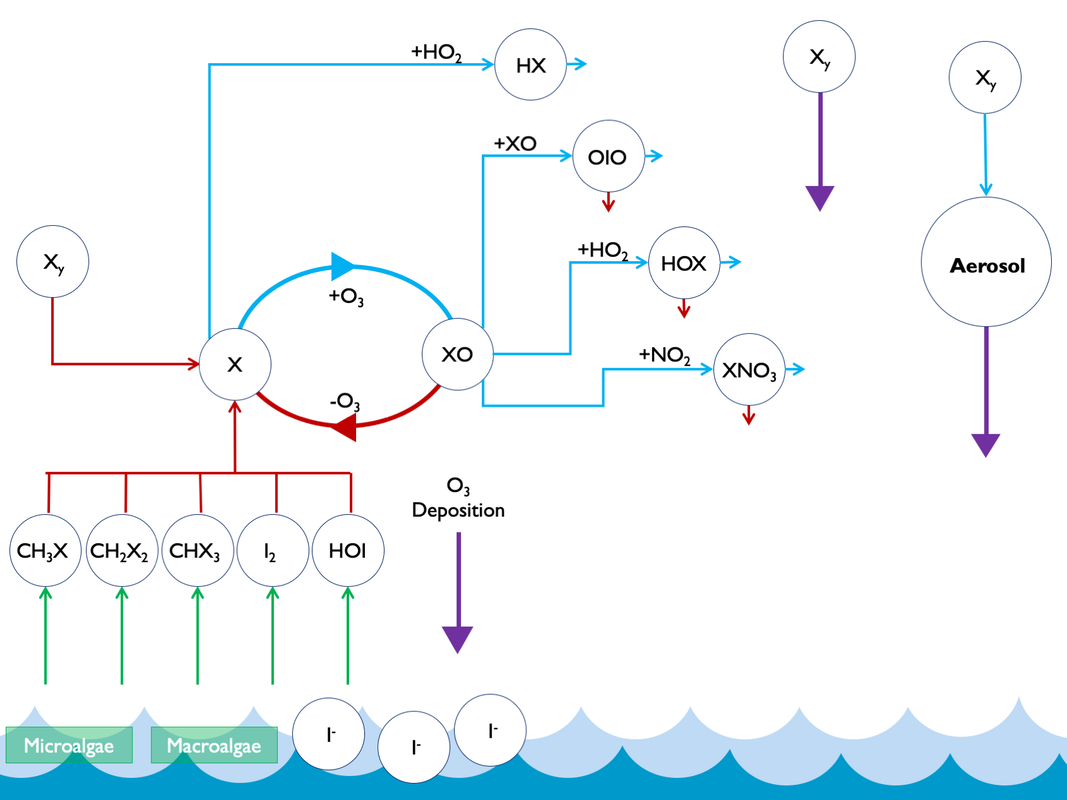
Research in this area has mostly focused on the atmospheric chemistry of bromine until recent years, partially because instruments were able to measure it at atmospherically relevant concentrations earlier than iodine. However, evidence suggests that iodine may play a notable role too. Iodine in the atmosphere predominantly originates from oceanic sources. Micro- and macro-algae turn iodine into more volatile species (e.g. CH3I, CH2I2) in seawater, which enter the atmosphere more easily. There are also abiotic production routes (producing HOI and I2). This emitted iodine then undergoes gas- and aerosol- phase chemistry, and is mostly returned back to the ocean through deposition. Some of this iodine is deposited on land, where it is an important bio-nutrient, and vital to global populations which have both historically and recently suffered too low an iodine content in their diets, leading to thyroid-related medical conditions such as Goitre (1, 2).
Research in this area has mostly focused on the atmospheric chemistry of bromine until recent years, partially because instruments were able to measure it at atmospherically relevant concentrations earlier than iodine. However, evidence suggests that iodine may play a notable role too. Iodine in the atmosphere predominantly originates from oceanic sources. Micro- and macro-algae turn iodine into more volatile species (e.g. CH3I, CH2I2) in seawater, which enter the atmosphere more easily. There are also abiotic production routes (producing HOI and I2). This emitted iodine then undergoes gas- and aerosol- phase chemistry, and is mostly returned back to the ocean through deposition. Some of this iodine is deposited on land, where it is an important bio-nutrient, and vital to global populations which have both historically and recently suffered too low an iodine content in their diets, leading to thyroid-related medical conditions such as Goitre (1, 2).
|
Whilst sources of iodine in the atmosphere were previously thought to be almost entirely organic (e.g. CH3I, CH2I2), laboratory work has shown one large source is abiotic sea-surface reactions involving ozone (3). This means that iodine emissions are linked to tropospheric ozone, which is both an air pollutant and climate gas. Global atmospheric models have demonstrated that this ozone-dependant source makes up the majority of emissions to the atmosphere (4). Figure 1 offers a simplified schematic of atmospheric cycles of iodine.
A changing atmosphere, from pre-industrial to present-day
The composition of the atmosphere has changed between pre-industrial revolution and present day. Two large chemical changes are increased emissions of both oxides of nitrogen (NOx) and volatile organic compounds (VOCs). NOx increases have been driven by high-temperature combustion, such as in power stations, which can combine nitrogen and oxygen. Industry and transport sectors have led to large direct emission of VOCs, such as from exhaust pipes of cars. The two ingredients may combine to produce more ozone in the troposphere. |
The increase in ozone from the preindustrial to present day leads to greater emissions of iodine. This occurs through abiotic sea-surface reactions that lead to HOI and I emission. The increased iodine then leads to more chemical destruction of ozone in the troposphere (Figure 1 and Reactions 1-3).
|
Whilst modelling work predicted this link between changes in atmospheric composition, increases in iodine emission and increases in ozone destruction (4), no historical observational records were known to confirm or disprove what the models said. However, within the last year, two studies have reported iodine concentrations in ice core records (5, 6). These two records, one in the Central Massif in France (Figure 2) and the other in Greenland, agree a three-fold increase since the 1950s. This is concurrent with the largest human-driven increases in tropospheric ozone. The overall trend is comparable to model predictions for the same period (4, 7, 8).
An unfolding story Excitingly, these recent observations lend additional weight to the theory that iodine acts to diminish the human-driven increase in ozone: a natural negative feedback. Although there are still many uncertainties, emerging evidence on iodine in the atmosphere suggests a notable role which has increased in response to human-driven changes. |
References
Note: Former ECG committee members William Bloss and Stephen Ball described ‘Iodine chemistry in the coastal marine atmosphere’ in an article in the ECG Bulletin, July 2009.
- Risher, John F., Samuel Keith, L., World Health Organisation and International Programme on Chemical Safety (2009). Iodine and inorganic iodides: human health aspects. World Health Organization. https://apps.who.int/iris/handle/10665/43579
- Andersson M, de Benoist B, Darnton-Hill I, Delange F, eds, World Health Organisation (2007). Iodine deficiency in Europe : a continuing public health problem. World Health Organization. https://apps.who.int/iris/handle/10665/43398
- Carpenter, L. J. et al., Nat. Geosci., 6, 108–111 (2013), doi:10.1038/ngeo1687
- Sherwen, T., Atmos. Chem. Phys., 16, 1161-1186 (2016).
- M. Legrand et al., Proc. Natl. Acad. Sci. USA., 115 (48) 12136-12141 (2018).
- C. A. Cuevas et al., Nat. Commun, 9, 1452 (2018).
- Prados-Roman, C., et al., Atmos. Chem. Phys., 15, 2215-2224 (2015).
- T. Sherwen et al., Atmos. Chem. Phys., 17, 1557-1569 (2017).
Note: Former ECG committee members William Bloss and Stephen Ball described ‘Iodine chemistry in the coastal marine atmosphere’ in an article in the ECG Bulletin, July 2009.