Synchrotron-based studies of green rust formation
Dr. Imad Ahmed, Dr. Sam Shaw, Ms. Gabriella Kakonyi and Prof. Liane G. Benning
ECG Bulletin July 2010
ECG Bulletin July 2010
The natural environment is rich in nanoparticulate mineral phases, such as iron and manganese oxides and oxyhydroxides, with unique chemical properties. Some of these minerals, for example Green Rusts, have the potential to be developed into a new generation of environmental remediation materials which could be utilized to clean up contaminated land. Advances in X-ray technologies at third -generation synchrotron sources (e.g. the Diamond Light Source) have helped to characterise the formation and crystallisation of highly reactive nanoparticles under simulated environmental conditions. In this article, Dr. Imad Ahmed, Dr. Sam Shaw, Ms. Gabriella Kakonyi and Prof. Liane G. Benning, describe how state of-
the-art in situ time-resolved synchrotron-based scattering and diffraction methods are used to determine the mechanisms and kinetics of green rust nanoparticle formation and growth.
the-art in situ time-resolved synchrotron-based scattering and diffraction methods are used to determine the mechanisms and kinetics of green rust nanoparticle formation and growth.
Introduction
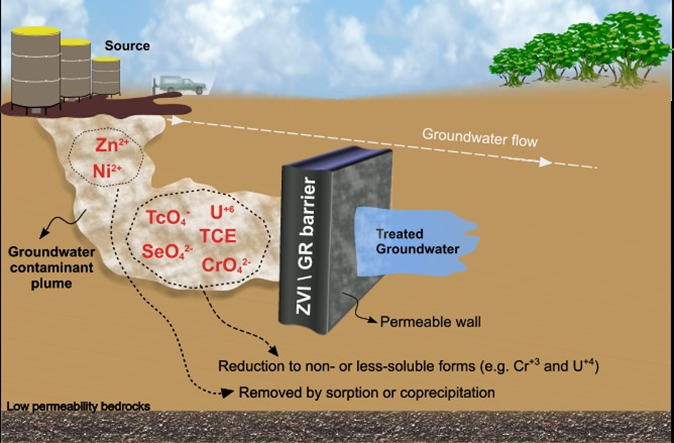
The remediation of contaminated land often requires the use of technologies (e.g. permeable reactive barriers or soil washing techniques (Bhandari et al., 2007) to clean up pollution caused by the legacy historical industrial activities (e.g. mining, chemical manufacturing etc.). The bioavailability and toxicity of many contaminants in the environment are controlled by their redox state. For example, CrO(sub>42– (i.e. Cr6+) is highly soluble and poses a significant threat to the environment, whereas Cr3+ is highly insoluble and poses a much reduced hazard. The reduction of redox-active inorganic and organic species for decontamination purposes is exploited in a number of remediation technologies including zero valent iron (ZVI) Permeable Reactive Barriers (PRB). In these systems, ZVI is used as a barrier where a large trench is dug at the edge of a contaminated land site to intercept contaminated groundwater moving from the site. Redox-active contaminants such as U6+ and Cr6+ are reduced as they interact with the Fe0 into less soluble and bioavailable forms (e.g. UO22+ to UO2(s)). In recent years, a large amount of research has also focused on the use of nanoparticulate remediation strategies, where the materials (e.g. nano-ZVI) are injected into a polluted subsurface aquifer (Figure 1) then move into the contamination plume and reductively decontaminate the groundwater (Zhang, 2003). For example, field trials in Florida (USA) reported significant reduction (~80%) in trichloroethene (TCE) groundwater concentrations upon the injection of nano-ZVI (Quinn et al., 2005).
The remediation of contaminated land often requires the use of technologies (e.g. permeable reactive barriers or soil washing techniques (Bhandari et al., 2007) to clean up pollution caused by the legacy historical industrial activities (e.g. mining, chemical manufacturing etc.). The bioavailability and toxicity of many contaminants in the environment are controlled by their redox state. For example, CrO(sub>42– (i.e. Cr6+) is highly soluble and poses a significant threat to the environment, whereas Cr3+ is highly insoluble and poses a much reduced hazard. The reduction of redox-active inorganic and organic species for decontamination purposes is exploited in a number of remediation technologies including zero valent iron (ZVI) Permeable Reactive Barriers (PRB). In these systems, ZVI is used as a barrier where a large trench is dug at the edge of a contaminated land site to intercept contaminated groundwater moving from the site. Redox-active contaminants such as U6+ and Cr6+ are reduced as they interact with the Fe0 into less soluble and bioavailable forms (e.g. UO22+ to UO2(s)). In recent years, a large amount of research has also focused on the use of nanoparticulate remediation strategies, where the materials (e.g. nano-ZVI) are injected into a polluted subsurface aquifer (Figure 1) then move into the contamination plume and reductively decontaminate the groundwater (Zhang, 2003). For example, field trials in Florida (USA) reported significant reduction (~80%) in trichloroethene (TCE) groundwater concentrations upon the injection of nano-ZVI (Quinn et al., 2005).
|
The development of nanoparticles based remediation technologies relies on the manufacture of materials which have suitable chemical and physical properties which allow them to adequately disperse within the contaminated plume and effectively remediate the contamination. Here we present research into green rust nanoparticles which have the potential to be utilized for environmental cleanup. However, their highly reactive nature makes them difficult to study using conventional techniques. Therefore we have developed a new synchrotron-based approach to studying the formation and stability of green rusts under the conditions they may be utilized in the environment.
|
What is green rust?
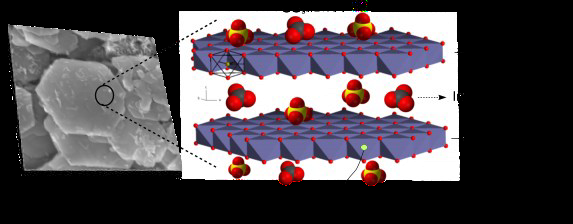
The Green Rust (GR) family of compounds have been suggested (Ruby et al., 2006b) as potential materials for use in reductive remediation technologies. The name green rust relates to their colour and formation from corroding iron. GR’s are mixed Fe2+/Fe3+ solid compounds with a Layered Double Hydroxide (LDH) structure. Each layer in the GR crystal is positively charged and composed of edge-sharing octahedrally coordinated Fe2+ and Fe3+ forming hydroxide sheets, which are intercalated with negatively charged anions and water molecules (Figure 2). GR’s have the general chemical formula:
The Green Rust (GR) family of compounds have been suggested (Ruby et al., 2006b) as potential materials for use in reductive remediation technologies. The name green rust relates to their colour and formation from corroding iron. GR’s are mixed Fe2+/Fe3+ solid compounds with a Layered Double Hydroxide (LDH) structure. Each layer in the GR crystal is positively charged and composed of edge-sharing octahedrally coordinated Fe2+ and Fe3+ forming hydroxide sheets, which are intercalated with negatively charged anions and water molecules (Figure 2). GR’s have the general chemical formula:
Various types of anionic species can be intercalated in the GR structure including SO42–, Cl–, CO32–, formate, and lactate (Drissi et al., 1995; Refait and Génin, 1993; Sabot et al., 2007; Sumoondur et al., 2009). Sulphate- and carbonate-containing GR (GRSO4 and GRCO3, respectively) are among the most studied forms, and have been found to occur in a number of natural (e.g. hydromorphic soils) and engineered environments (e.g. corrosion of steel in seawater, Olowe and Genin, 1991). In these environments, green rusts (also known by its mineral name ‘fougerite’, Génin et al., 2001) tend to form at the interface between the oxic and anoxic zones where both Fe2+ and Fe3+ can coexist leading to the formation of mixed oxidation state compounds.
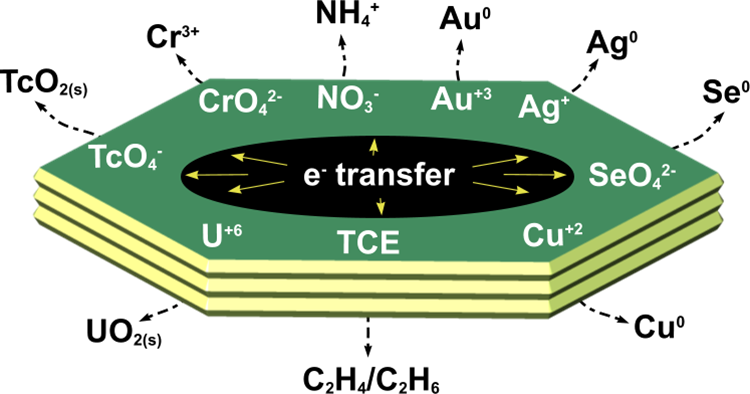
Recent research has shown that the presence of highly reactive Fe2+ within the GR structure give these materials a large capacity to reduce a variety of mobile inorganic and organic contaminants (Legrand et al., 2004; Myneni et al., 1997; O’Loughlin et al., 2003). The reduction of inorganic species leads, in many cases, to the formation of highly insoluble phases that are non- mobile and less bioavailable in the environment. For example, GR has been shown to reduce the soluble uranyl ions (UO22+) into insoluble UO2(s) nanoparticles (O’Loughlin et al., 2003). Also, the reductive transformation of chlorinated hydrocarbons (e.g. TCE and DCM) leads to dechlorination and breakdown of these toxic pollutants (O’Loughlin and Burris, 2004). Other examples pertaining to the reductive potential of GR materials are summarised in Figure 3. These unique redox properties make GR a potential candidate for use as a ‘reactive redox barrier’ material for environmental prevention and cleanup technologies such as PRB systems.
|
Due to their high Fe2+ content, GR’s are highly reactive reductants, but this also means that they transform into ferric phases (e.g. goethite) within minutes of exposure to air. This reactivity caused GR’s to go unnoticed in many natural and geo-environmental systems for many years, and leads to great difficulties in handling, sampling and characterizing these phases. Similar to other highly redox-reactive phases such as mackinawite (nominally “FeS”), characterization of GR using conventional ex situ techniques such as X-ray powder diffraction (XRD) and infrared spectroscopy requires sample pre-treatment procedures (e.g. drying, freeze-drying and washing) is difficult. We found that GRSO4 can oxidize during ageing for several days in an H2/N2-glove box with an O2 content as low as ≤ 0.005%. This makes studying the formation, transformation pathways and the stability of air-sensitive GR via conventional ex situ techniques challenging, time consuming and in some cases inaccurate.
|
In addition, identification of short-lived (minutes to hours) precursors or intermediate phases in any reaction is even more challenging using traditional ex situ methods. This is often because the precursors/intermediates are either amorphous or semi-crystalline and usually highly sensitive to physicochemical changes in the reacting solution (e.g. pH, redox potential or mixing rates). In the next section, we describe a novel in situ approach for studying the formation and transformation of highly reactive phases, and its use in characterising the formation and transformation of GR.
Characterisation of GR using in situ X-ray scattering technique
To overcome the difficulties of studying GR, we developed a synchrotron-based in situ approach for characterizing the formation of air sensitive nanoparticles. This approach makes use of a chemostat reactor combined with in situ time resolved X-ray scattering measurements. The Small- and Wide angle X-ray Scattering (SAXS/WAXS) experiments were carried out on beamline station MPW6.2 of the Synchrotron Radiation Source (SRS), Daresbury Laboratory, UK and beamline I22 of the Diamond Light Source (DLS), Oxford, UK. The WAXS measurements collected at scattering angles > 5° provides data on the intramolecular and interatomic correlations for crystalline or semi-crystalline structures. The SAXS (scattering angle < 5°) data provides information on larger features (as large as ~ 200 nm) in solid samples and thus it is a useful technique in studying ordering on the meso- and nano-length scales, such as particle size and morphology. Figure 4 shows a schematic diagram of the experimental setup. A chemostat system, which allowed precise control and measurement of pH and redox potential (Eh) was used to synthesise the GR under anaerobic conditions. The solutions or dilute suspensions were constantly circulated through a closed loop consisting of a quartz capillary (1.5 mm ID, 10 µm wall) connected to the reaction vessel via Teflon tubing. The quartz capillary was aligned with the synchrotron X-ray beam (λ = 1.0 Å) allowing collection of time-resolved WAXS and SAXS data at selected time interval (typically 120 sec), for up to 18 hours. This was done simultaneous with the time resolved monitoring of the chemical data (i.e. pH, redox potential (Eh) and volume of reactants added, which were recorded via an automated control unit attached to the chemostat system. As shown in Figure 4, the WAXS detector was positioned to cover a wide angular range of 5 – 65° from normal to the X-ray beam. The SAXS detector positioned to collect data at ≤ 5° and was positioned at the end of a 3-8 meter camera under vacuum.
To overcome the difficulties of studying GR, we developed a synchrotron-based in situ approach for characterizing the formation of air sensitive nanoparticles. This approach makes use of a chemostat reactor combined with in situ time resolved X-ray scattering measurements. The Small- and Wide angle X-ray Scattering (SAXS/WAXS) experiments were carried out on beamline station MPW6.2 of the Synchrotron Radiation Source (SRS), Daresbury Laboratory, UK and beamline I22 of the Diamond Light Source (DLS), Oxford, UK. The WAXS measurements collected at scattering angles > 5° provides data on the intramolecular and interatomic correlations for crystalline or semi-crystalline structures. The SAXS (scattering angle < 5°) data provides information on larger features (as large as ~ 200 nm) in solid samples and thus it is a useful technique in studying ordering on the meso- and nano-length scales, such as particle size and morphology. Figure 4 shows a schematic diagram of the experimental setup. A chemostat system, which allowed precise control and measurement of pH and redox potential (Eh) was used to synthesise the GR under anaerobic conditions. The solutions or dilute suspensions were constantly circulated through a closed loop consisting of a quartz capillary (1.5 mm ID, 10 µm wall) connected to the reaction vessel via Teflon tubing. The quartz capillary was aligned with the synchrotron X-ray beam (λ = 1.0 Å) allowing collection of time-resolved WAXS and SAXS data at selected time interval (typically 120 sec), for up to 18 hours. This was done simultaneous with the time resolved monitoring of the chemical data (i.e. pH, redox potential (Eh) and volume of reactants added, which were recorded via an automated control unit attached to the chemostat system. As shown in Figure 4, the WAXS detector was positioned to cover a wide angular range of 5 – 65° from normal to the X-ray beam. The SAXS detector positioned to collect data at ≤ 5° and was positioned at the end of a 3-8 meter camera under vacuum.
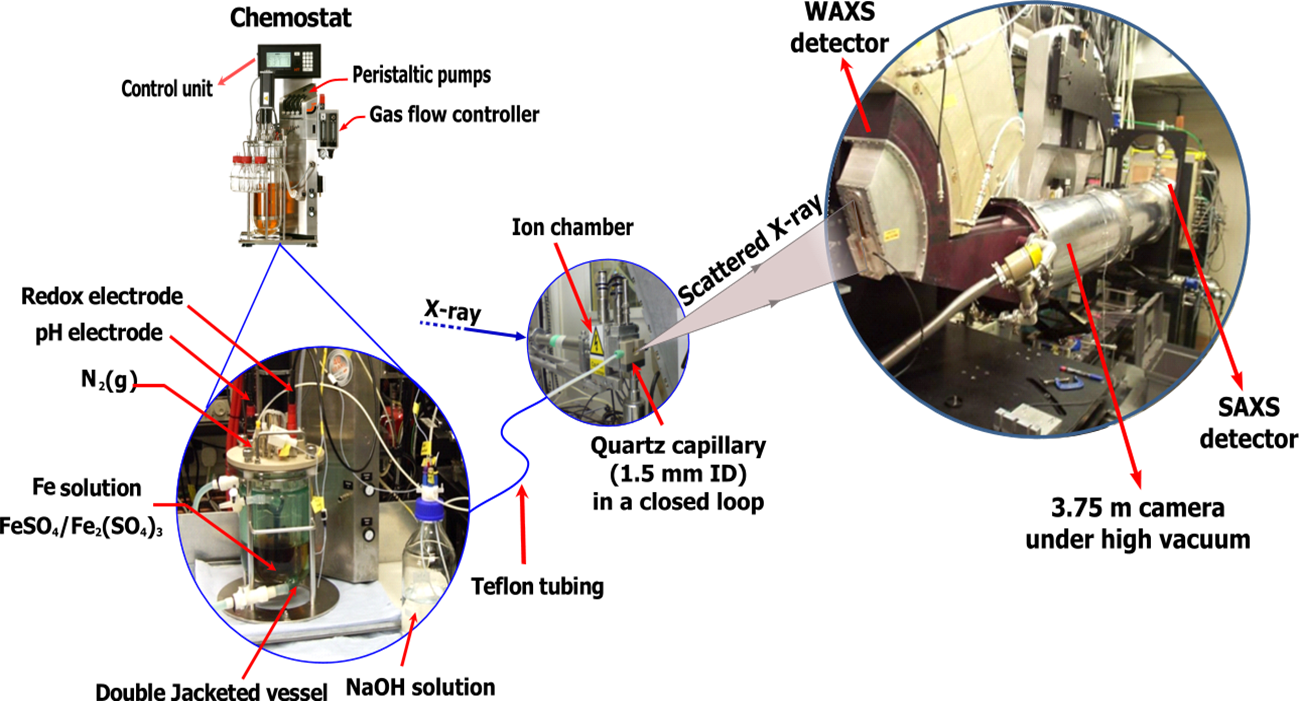
Figure 4: Schematic diagram of the experimental setup used for the synthesis of GR and the simultaneous collection of electrochemical and WAXS/SAXS data. The contents of the double jacketed reaction vessel were constantly circulated through a quartz capillary mounted in-line with the incident synchrotron X-ray beam
Formation of green rust
Green rust was synthesised by induced hydrolysis under strict anoxic conditions. The reaction was initiated by the progressive addition of a basic solution to a solution containing dissolved Fe2+ and Fe3+. Different GR forms can be synthesised using this approach. For example, GRSO4 was synthesised by mixing Fe2+ and Fe3+ sulphate and adding NaOH. If Na2CO3 was used instead of NaOH, GRSO4 formed at pH < 8.5, and was transformed completely into GRCO3 at pH above 9.
Green rust was synthesised by induced hydrolysis under strict anoxic conditions. The reaction was initiated by the progressive addition of a basic solution to a solution containing dissolved Fe2+ and Fe3+. Different GR forms can be synthesised using this approach. For example, GRSO4 was synthesised by mixing Fe2+ and Fe3+ sulphate and adding NaOH. If Na2CO3 was used instead of NaOH, GRSO4 formed at pH < 8.5, and was transformed completely into GRCO3 at pH above 9.
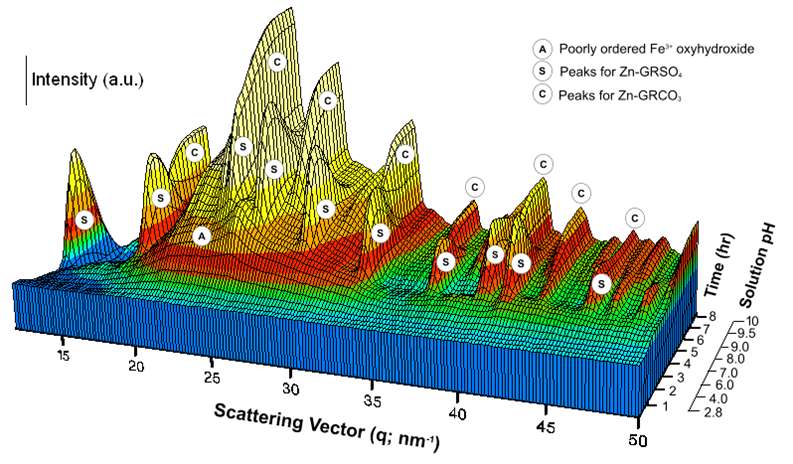
Zn-containing GRSO4 (Zn-GRSO4) and GRCO3 (Zn-GRCO3) phases were synthesised by coprecipitation following the method described by Ahmed et al., (2008). A 3D representation of the time-resolved WAXS patterns from the experiment forming Zn-GR is shown in Figure 5. Analyzing these data vs reaction time allowed the quantification of the complex reaction sequences that are controlled by the changes in the solution chemistry (e.g. pH). In this way, GR formation and transformation pathways as well as the mechanisms for each reaction step were derived. In Ahmed et al., (2008), we have shown that schwertmannite (an Fe3+ oxyhydroxysulphate; Fe8O8(OH)4.5(SO4)1.75) was a precursor of Zn-GRSO4, which formed at pH 6.9 - 9.5 (Figure 5). Beyond pH ~8.3, HCO3– (from the addition of Na2CO3) dissociated into CO32– and exchanged for the intercalated SO42– in the interlayer of Zn-GRSO4, leading to the formation of Zn-GRCO3.
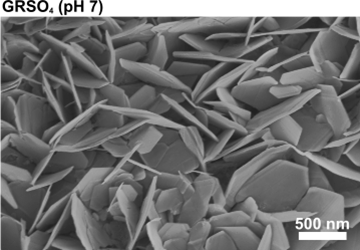
As shown in Figure 6, both GRSO4 and GRCO3 (freshly synthesised by coprecipitation) exhibit hexagonal plate-like particle morphologies with an average edge length of ~250 nm. However, GRCO3 possesses almost three times the thickness (~ 80 nm) of GRSO4 (Ahmed et al., 2008).
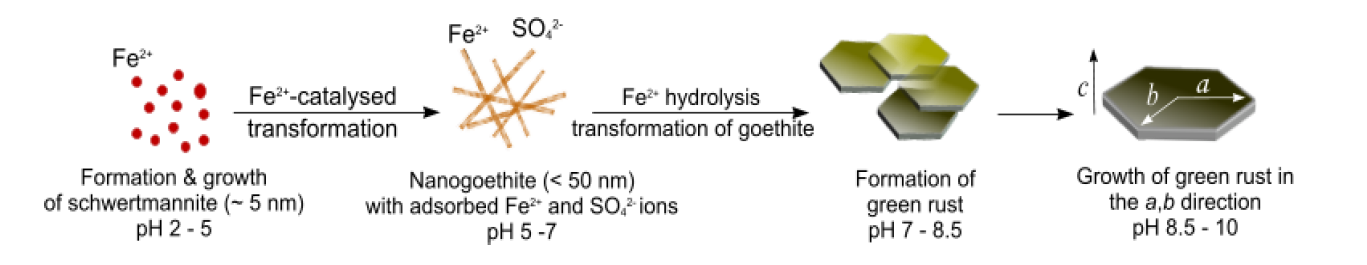
In a more recent paper (Ahmed et al., 2010), we demonstrated that schwertmannite (~ 5 nm) and nanogoethite (< 50 nm) precede the formation of GRSO4. Schwertmannite formed at pH 2.8 – 4.5 by the hydrolysis of Fe3+ and then transformed within ~1 h into nanogoethite at pH 5.5 – 6. The transformation reaction of schwertmannite into goethite in most environmental systems is known to be very slow and requires several months to complete. We have suggested that in the presence of Fe2+ this reaction is catalysed via the surface adsorption of Fe2+ ions onto the schwertmannite nanoparticles. This initiates an electron transfer between the Fe3+ in the solid phase and the surface-bound Fe2+ ions. This reaction decreases the stability of the schwertmannite crystal leading to structural re-arrangements and finally formation of the more stable goethite phase. With increasing pH the goethite transforms gradually into GRSO4. At pH > 7, the solubility of Fe2+ decreases which leads to the incorporation of Fe2+ ions in oxyhydroxide structure and the gradual formation of mixed Fe2+/Fe3+ octahedral sheets (primary building units of GR) whose excess positive charge is stabilised by the adsorption of sulphate ions. As Fe2+ hydrolysis continues to occur, the formation and growth of these Fe2+/Fe3+ sheets continue until GR crystals are well formed. The GR crystals continue to grow in the a,b crystallographic direction with increasing pH up to 10. These processes are summarised schematically in Figure 7.
Outlook
Similar to ZVI, GR oxidizes quickly when in contact with chemical species carrying electron-deficient centres (e.g. O2 and NO3–) which limit the longevity of the redox barrier. However, more can be done to extend the longevity of GR-containing barrier. For example, it has been demonstrated that the presence of trace amounts of dissolved phosphate or silicate increased the GR stability towards oxidative transformations. This was explained by the adsorption of these species at the lateral faces of GR (Ruby et al., 2006a). It is also known that the type of intercalated anions is critical to defining the chemical stability, particle size and shape of the GR material. For example, chloride GR is less stable than the corresponding sulphate and carbonate forms of GR and its formation/stability in the natural environment seems unlikely.
Similar to ZVI, GR oxidizes quickly when in contact with chemical species carrying electron-deficient centres (e.g. O2 and NO3–) which limit the longevity of the redox barrier. However, more can be done to extend the longevity of GR-containing barrier. For example, it has been demonstrated that the presence of trace amounts of dissolved phosphate or silicate increased the GR stability towards oxidative transformations. This was explained by the adsorption of these species at the lateral faces of GR (Ruby et al., 2006a). It is also known that the type of intercalated anions is critical to defining the chemical stability, particle size and shape of the GR material. For example, chloride GR is less stable than the corresponding sulphate and carbonate forms of GR and its formation/stability in the natural environment seems unlikely.
We are currently testing a range of GR compounds in the laboratory to characterise their effectiveness at reducing a range of contaminates including CrO42-, SeO42- and UO22+. Our aim is to understand whether any of the GR compounds we have synthesised will be suitable for decontaminating a
range of industrial effluents. This includes studies of the GR clean up of simulated radionuclides waste streams to determine if these materials could be used to immobilise redox sensitive radionuclides (e.g. U) in these polluted effluents.
Web links
Mineral databases:
http://www.mindat.org/index.php
http://www.galleries.com/minerals/by-name.htm
Diamond Light Source: http://www.diamond.ac.uk/
Acknowledgments
We would like to gratefully acknowledge the Natural Environment Research Council (NERC; grant No. NE/D014026/1) for funding this research. We also thank the STFC for the provision of facilities at the Daresbury Synchrotron Radiation Laboratory (SRS grant No. 48163) and Diamond Light Source (SRS grant No. SM883). Thanks to Dr. Nick Terrill and his team at the I22-non-crystalline-diffraction station of the Diamond Light Source for the technical support in undertaking the WAXS and SAXS experiments. Thanks are also extended to the technical support at the Leeds Electron microscopy and Spectroscopy centre (LEMAS) in the collection and analysis of SEM images and to colleagues of the Shaw and Benning groups, specifically Aryani Sumoondur for fruitful discussions throughout this project.
Mineral databases:
http://www.mindat.org/index.php
http://www.galleries.com/minerals/by-name.htm
Diamond Light Source: http://www.diamond.ac.uk/
Acknowledgments
We would like to gratefully acknowledge the Natural Environment Research Council (NERC; grant No. NE/D014026/1) for funding this research. We also thank the STFC for the provision of facilities at the Daresbury Synchrotron Radiation Laboratory (SRS grant No. 48163) and Diamond Light Source (SRS grant No. SM883). Thanks to Dr. Nick Terrill and his team at the I22-non-crystalline-diffraction station of the Diamond Light Source for the technical support in undertaking the WAXS and SAXS experiments. Thanks are also extended to the technical support at the Leeds Electron microscopy and Spectroscopy centre (LEMAS) in the collection and analysis of SEM images and to colleagues of the Shaw and Benning groups, specifically Aryani Sumoondur for fruitful discussions throughout this project.
References
Ahmed, I. A. M.; Benning, L. G.; Kakonyi, G.; Sumoondur, A. D.; Terrill, N. J.; Shaw, S., 2010. Formation of Green Rust Sulfate: A combined in situ time-resolved X-ray scattering and electrochemical study. Langmuir 26, 6593-6603
Ahmed, I. A. M.; Shaw, S.; Benning, L. G., 2008. Formation of hydroxysulphate and hydroxycarbonate green rusts in the presence of zinc using time-resolved in situ small and wide angle X-ray scattering. Mineralogical Magazine 72, 1-4.
Bhandari, A.; Rao, Y. S.; Pascale, C.; Say Kee, O., 2007. Remediation Technologies for Soils and Groundwater. American Society of Civil Engineers.
Drissi, S. H.; Refait, P.; Abdelmoula, M.; Génin, J. M. R., 1995. The preparation and thermodynamic properties of Fe(II)-Fe(III) hydroxide-carbonate (Green Rust 1): Pourbaix diagram of iron in carbonate-containing aqueous media. Corrosion Science 37, 2025-2041.
Génin, J. M. R.; Refait, P.; Bourrie, G.; Abdelmoula, M.; Trolard, F., 2001. Structure and stability of the Fe(II)-Fe(III) Green Rust "Fougerite" mineral and its potential for reducing pollutants in soil solutions. Applied Geochemistry 16, 559-570.
Legrand, L.; El Figuigui, A.; Mercier, F.; Chausse, A., 2004. Reduction of aqueous chromate by Fe(II)/Fe(III) carbonate green rust: Kinetic and mechanistic studies. Environmental Science & Technology 38, 4587-4595.
Myneni, S. C. B.; Tokunaga, T. K.; Brown, G. E., 1997. Abiotic selenium redox transformations in the presence of Fe(II,III) oxides. Science 278, 1106-1109.
O’Loughlin, E. J.; Burris, D. R., 2004. Reduction of halogenated ethanes by green rust. Environmental Toxicology and Chemistry 23, 41-48.
O’Loughlin, E. J.; Kelly, S. D.; Cook, R. E.; Csencsits, R.; Kemner, K. M., 2003. Reduction of Uranium(VI) by mixed iron(II)/iron(III) hydroxide (Green Rust): Formation of UO2 nanoparticles. Environmental Science & Technology 37, 721-727.
Olowe, A. A.; Genin, J. M. R., 1991. The mechanism of oxidation of ferrous hydroxide in sulphated aqueous media: Importance of the initial ratio of the reactants. Corrosion Science 32, 965-984.
Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.-S.; Gavaskar, A.; Holdsworth, T., 2005. Field Demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environmental Science & Technology 39, 1309-1318.
Refait, P.; Abdelmoula, M.; Genin, J. M. R.; Jeannin, M., 2006. Synthesis and characterisation of the Fe(II-III) hydroxy-formate green rust. Hyperfine Interactions 167, 717-722.
Refait, P.; Génin, J. M. R., 1993. The oxidation of ferrous hydroxide in chloride-containing aqueous media and pourbaix diagrams of green rust one. Corrosion Science 34, 797-819.
Ruby, C.; Géhin, A.; Aissa, R.; Ghanbaja, J.; Abdelmoula, M.; Génin, J. M. R., 2006a. Chemical stability of hydroxysulphate green rust synthesised in the presence of foreign anions: carbonate, phosphate and silicate. Hyperfine Interactions 167, 803-807.
Ruby, C.; Upadhyay, C.; Géhin, A.; Ona-Nguema, G.; Génin, J. M. R., 2006b. In situ redox flexibility of Fe II-III oxyhydroxycarbonate Green Rust and fougerite. Environmental Science & Technology 40, 4696-4702.
Sabot, R.; Jeannin, M.; Gadouleau, M.; Guo, Q.; Sicre, E.; Refait, P., 2007. Influence of lactate ions on the formation of rust. Corrosion Science 49, 1610-1624.
Sumoondur, A. D.; Shaw, S.; Benning, L. G., 2009. Formation of lactate intercalated Green Rust via the reductive dissolution of ferrihydrite. Geochimica et Cosmochimica Acta 73, A1291.
Trolard, F.; Génin, J. M. R.; Abdelmoula, M.; Bourrie, G.; Humbert, B.; Herbillon, A., 1997. Identification of a Green Rust mineral in a reductomorphic soil by Mössbauer and Raman spectroscopies. Geochimica et Cosmochimica Acta 61, 1107-1111.
Zhang, W.-x., 2003. Nanoscale Iron Particles for Environmental Remediation: An Overview. Journal of Nanoparticle Research 5, 323-332.
Ahmed, I. A. M.; Benning, L. G.; Kakonyi, G.; Sumoondur, A. D.; Terrill, N. J.; Shaw, S., 2010. Formation of Green Rust Sulfate: A combined in situ time-resolved X-ray scattering and electrochemical study. Langmuir 26, 6593-6603
Ahmed, I. A. M.; Shaw, S.; Benning, L. G., 2008. Formation of hydroxysulphate and hydroxycarbonate green rusts in the presence of zinc using time-resolved in situ small and wide angle X-ray scattering. Mineralogical Magazine 72, 1-4.
Bhandari, A.; Rao, Y. S.; Pascale, C.; Say Kee, O., 2007. Remediation Technologies for Soils and Groundwater. American Society of Civil Engineers.
Drissi, S. H.; Refait, P.; Abdelmoula, M.; Génin, J. M. R., 1995. The preparation and thermodynamic properties of Fe(II)-Fe(III) hydroxide-carbonate (Green Rust 1): Pourbaix diagram of iron in carbonate-containing aqueous media. Corrosion Science 37, 2025-2041.
Génin, J. M. R.; Refait, P.; Bourrie, G.; Abdelmoula, M.; Trolard, F., 2001. Structure and stability of the Fe(II)-Fe(III) Green Rust "Fougerite" mineral and its potential for reducing pollutants in soil solutions. Applied Geochemistry 16, 559-570.
Legrand, L.; El Figuigui, A.; Mercier, F.; Chausse, A., 2004. Reduction of aqueous chromate by Fe(II)/Fe(III) carbonate green rust: Kinetic and mechanistic studies. Environmental Science & Technology 38, 4587-4595.
Myneni, S. C. B.; Tokunaga, T. K.; Brown, G. E., 1997. Abiotic selenium redox transformations in the presence of Fe(II,III) oxides. Science 278, 1106-1109.
O’Loughlin, E. J.; Burris, D. R., 2004. Reduction of halogenated ethanes by green rust. Environmental Toxicology and Chemistry 23, 41-48.
O’Loughlin, E. J.; Kelly, S. D.; Cook, R. E.; Csencsits, R.; Kemner, K. M., 2003. Reduction of Uranium(VI) by mixed iron(II)/iron(III) hydroxide (Green Rust): Formation of UO2 nanoparticles. Environmental Science & Technology 37, 721-727.
Olowe, A. A.; Genin, J. M. R., 1991. The mechanism of oxidation of ferrous hydroxide in sulphated aqueous media: Importance of the initial ratio of the reactants. Corrosion Science 32, 965-984.
Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.-S.; Gavaskar, A.; Holdsworth, T., 2005. Field Demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environmental Science & Technology 39, 1309-1318.
Refait, P.; Abdelmoula, M.; Genin, J. M. R.; Jeannin, M., 2006. Synthesis and characterisation of the Fe(II-III) hydroxy-formate green rust. Hyperfine Interactions 167, 717-722.
Refait, P.; Génin, J. M. R., 1993. The oxidation of ferrous hydroxide in chloride-containing aqueous media and pourbaix diagrams of green rust one. Corrosion Science 34, 797-819.
Ruby, C.; Géhin, A.; Aissa, R.; Ghanbaja, J.; Abdelmoula, M.; Génin, J. M. R., 2006a. Chemical stability of hydroxysulphate green rust synthesised in the presence of foreign anions: carbonate, phosphate and silicate. Hyperfine Interactions 167, 803-807.
Ruby, C.; Upadhyay, C.; Géhin, A.; Ona-Nguema, G.; Génin, J. M. R., 2006b. In situ redox flexibility of Fe II-III oxyhydroxycarbonate Green Rust and fougerite. Environmental Science & Technology 40, 4696-4702.
Sabot, R.; Jeannin, M.; Gadouleau, M.; Guo, Q.; Sicre, E.; Refait, P., 2007. Influence of lactate ions on the formation of rust. Corrosion Science 49, 1610-1624.
Sumoondur, A. D.; Shaw, S.; Benning, L. G., 2009. Formation of lactate intercalated Green Rust via the reductive dissolution of ferrihydrite. Geochimica et Cosmochimica Acta 73, A1291.
Trolard, F.; Génin, J. M. R.; Abdelmoula, M.; Bourrie, G.; Humbert, B.; Herbillon, A., 1997. Identification of a Green Rust mineral in a reductomorphic soil by Mössbauer and Raman spectroscopies. Geochimica et Cosmochimica Acta 61, 1107-1111.
Zhang, W.-x., 2003. Nanoscale Iron Particles for Environmental Remediation: An Overview. Journal of Nanoparticle Research 5, 323-332.