Sorption of metals onto microplastics
Common polymers used to make plastics, such as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS), were not considered important sorbents of environmental pollutants until recently. However, current research has shown that the microplastics may accumulate chemical pollutants on their surfaces through physical and chemical sorption processes. Most research thus far has focussed on the sorption of organic pollutants such as pharmaceuticals; however, a few key studies have demonstrated that microplastics can sorb metals (1-5). This research, although in its infancy, is essential to understanding both the influence of microplastics on the environmental fate of inorganic pollutants, and of the potential impacts of metal-loaded microplastics on organisms.
|
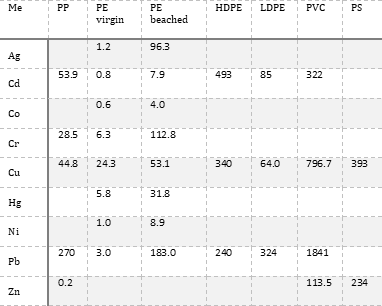
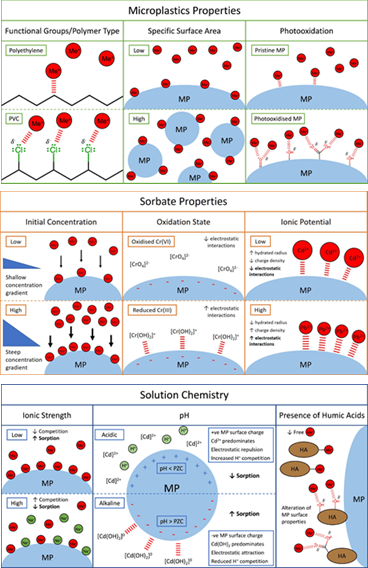
In the experimental metal-microplastic systems shown in Table 1, the distribution coefficient (KD) quantifies the partitioning of the metal between aqueous and microplastic-sorbed or solid phases, with a higher KD value indicating a higher proportion of sorbed metal relative to dissolved metal at equilibrium. Experimentally determined KD values vary greatly both within and between microplastic types. Microplastic composition and metal type account for some, but not all, of the variation. Sorption is a complicated process, and is influenced by a) the physicochemical properties of the microplastics (specific surface area, functional groups, degree of surface photo-oxidation, zeta potential at experimental pH), b) the chemical properties of the metal (concentration, oxidation state, ionic potential), c) the chemistry of the experimental medium (pH, ionic strength, presence of humic substances), and d) the physical conditions of the experiment (contact time, solid-to-liquid ratio, temperature) (5-8). Key factors are summarised in Figure 1. |
Table 1. Mean distribution coefficients (KD; mL g-1) quantifying sorption of various metals onto microplastics. Values are from available literature data (1-5). Me = metal, PP = polypropylene, PE = polyethylene, HDPE = high-density polyethylene, LDPE = low-density polyethylene, PVC = polyvinyl chloride, PS = polystyrene. |
|
Although current research has not been systematic, and data gaps exist, some tentative conclusions about metal sorption onto microplastics may be drawn. The composition of microplastics influences their capacity to sorb metals. PVC contains regularly distributed C-Cl bonds, which are polar due to the high electronegativity of chlorine. Chlorine atoms on the polymer surface create localised centres of partial negative charge, resulting in stronger electrostatic interactions between the microplastic surface and cationic metals (5). The relative order of metal sorption is different for each plastic type, however, meaning that functional groups are not the only control. Microplastics with a high specific surface area (SSA) provide a greater number of potential sorption sites relative to mass (8). As plastics degrade into smaller fragments over time, their specific surface area, and consequently sorption capacity for pollutants, increases.
Under exposure to ultra-violet radiation, photo-oxidative microplastic weathering takes place. This increases the surface density of oxygen-containing functional groups, which are thought to increase the relative strength of physical sorbent-sorbate interactions, due to the electronegativity of oxygen-creating localised centres (Figure 1) (9). Biofilm formation on the surfaces of microplastics is also thought to influence sorption capacity over time. Colonising bacteria secrete extracellular polymeric substances, such as polysaccharides, forming a biofilm on the microplastic surface. Biofilms change the surface properties of the microplastics, for example by introducing new hydroxyl, amine, and carboxylic acid groups (10).
|
Solution chemistry can alter both microplastic surface properties and the speciation of the metal, with pH perhaps the most influential factor (5-9). Under strongly acidic conditions, there is increased competition between cationic metal ions and hydronium (H3O+) ions for sorption sites on the microplastics. The zeta potential of the microplastic surfaces depends on solution pH. The net surface charge of the microplastics becomes increasingly negative as pH increases and, below the point of zero charge (PZC), increasingly positive as pH decreases. Figure 1 shows the example of Cd, which exists in solution almost entirely as divalent Cd2+ ions at pH < 6. As pH increases, CdOH+ (pH 6-7) and Cd(OH)2 (pH > 8) ions predominate. Where solution pH is below the PZC of the microplastics, electrostatic repulsion between the positively charged microplastic surface and the cationic Cd2+ ions are high. Highly ionic solutions, such as seawater, contain high concentrations of competitor ions, such as sodium (Na+), which can also sorb to the microplastics, occupying sorption sites and decreasing the sorption capacity for metals. The presence of humic acids (HAs) may increase or decrease metal sorption to microplastics. HAs are high molecular weight organic macromolecules with heterogeneous branching structures and oxygen-containing functional groups. HAs may sequester aqueous metals, reducing free metal ion concentration, and consequently decreasing sorption (7). Contrarily, HAs may themselves sorb onto microplastics, altering their surface properties by introducing new oxygen-containing functional groups (6).
Potentially toxic metals, including lead (Pb) and cadmium (Cd), show limited potential to sorb onto pristine microplastic surfaces. However, current evidence suggests that the sorption capacity of microplastics for pollutants increases over time, due to fragmentation, photo-oxidation, and biofilm formation, and it is therefore a multifaceted environmental phenomenon that requires careful, systematic study. Microplastics may act as vectors to transport metals into new environmental spheres or into organisms.
During wastewater treatment, microplastics are removed from the wastewater with a very high efficiency (88-94%). However, the vast majority subsist in sewage sludge (12), which is commonly applied to agricultural land as a fertiliser, presenting a pathway for metal-loaded microplastics to be transported into soils. Upon ingestion, the acidic conditions in the digestive tracts of organisms may facilitate the desorption of bound metals, potentially increasing their bioavailability. In soils, plants acidify the rhizosphere, and release root exudates during nutrient uptake. Implications for the mobility and bioavailability of microplastic-bound phytonutrients, especially micronutrients, and phytotoxins, present an important knowledge gap. As plastic production continues to increase year-on-year with no sign of slowing down, microplastics are predicted to continue to accumulate in terrestrial and aquatic environments.
- Holmes, L.A., Turner, A. and Thompson, R.C., (2012). Adsorption of trace metals to plastic resin pellets in the marine environment. Environmental Pollution, 160, pp.42-48.
- Turner, A. and Holmes, L.A., (2015). Adsorption of trace metals by microplastic pellets in fresh water. Environmental Chemistry, 12(5), pp.600-610.
- Brennecke, D. et al. (2016). Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coastal and Shelf Science, 178, pp.189-195.
- Gao, F., Li et al. (2019). Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Marine Pollution Bulletin, 144, pp.61-67.
- Zou, J., Liu, X., Zhang, D. and Yuan, X., (2020). Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere, 248, p.126064.
- Guo, X., Hu, G., Fan, X. and Jia, H., (2020). Sorption properties of cadmium on microplastics: the common practice experiment and a two-dimensional correlation spectroscopic study. Ecotoxicology and Environmental Safety, 190, p.110118.
- Zhou, Y. et al. (2020). Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Research, 184, p.116209.
- Wang, F. et al. (2019). Adsorption characteristics of cadmium onto microplastics from aqueous solutions. Chemosphere, 235, pp.1073-1080.
- Liu, S. et al. (2021). Interactions Between Microplastics and Heavy Metals in Aquatic Environments: A Review. Frontiers in Microbiology, 12, p.652520.
- Wang, J., Guo, X. and Xue, J. (2021). Biofilm-Developed Microplastics As Vectors of Pollutants in Aquatic Environments. Environmental Science & Technology, 55, pp.12780-12790.
- Rouff, A.A. (2012). Sorption of chromium with struvite during phosphorus recovery. Environmental Science & Technology, 46(22), pp.12493-12501.
- Iyare, P.U., Ouki, S.K. and Bond, T. (2020). Microplastics removal in wastewater treatment plants: Environ. Sci.: Water Res. Technol.,6, pp.2664-2675