Porous boron nitride: an effective adsorbent for carbon dioxide capture
Hojatisaeidi Fereshteh (London South Bank University, [email protected]), Mureddu Mauro (Sotacarbo SpA), Dessì Federica (Sotacarbo SpA), Pettinau Alberto (Sotacarbo SpA), Durand Geraldine (London South Bank University and The Welding Institute) and Saha Basudeb (London South Bank University)
ECG Bulletin July 2020
ECG Bulletin July 2020
Carbon capture and storage (CCS) will be essential to implementing the Paris Agreement to limit global warming to 1.5 °C above pre-industrial levels (1). Adsorption-based materials are associated with lower energy consumption in carbon capture technologies compared with conventional solvent-based materials. These adsorbents, which are mostly porous solids, have been applied to CCS under a wide range of temperature and pressure conditions (2). Porous boron nitride (BN) materials are a promising class of solid adsorbents for carbon capture applications due to their low cost and ease of manufacture.
Global greenhouse gas emission
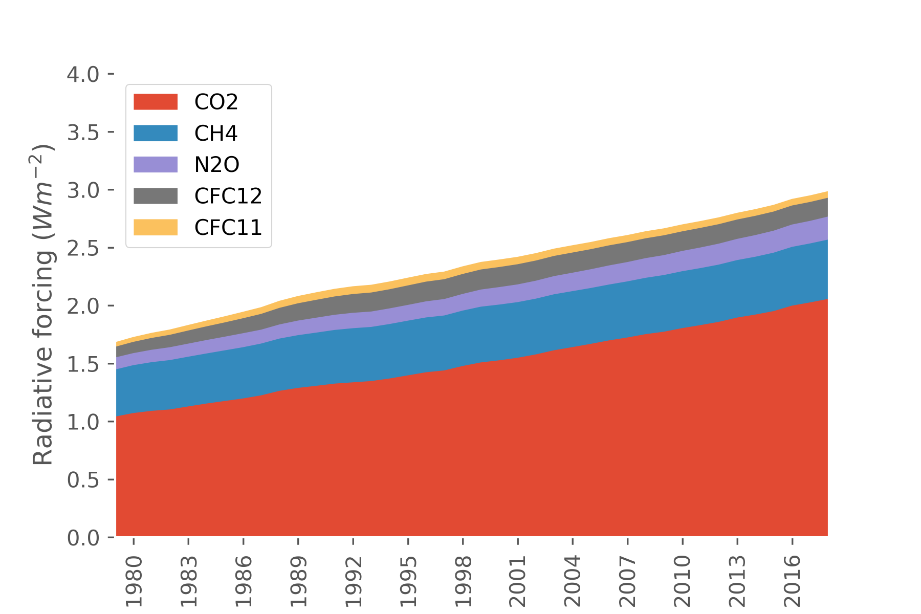
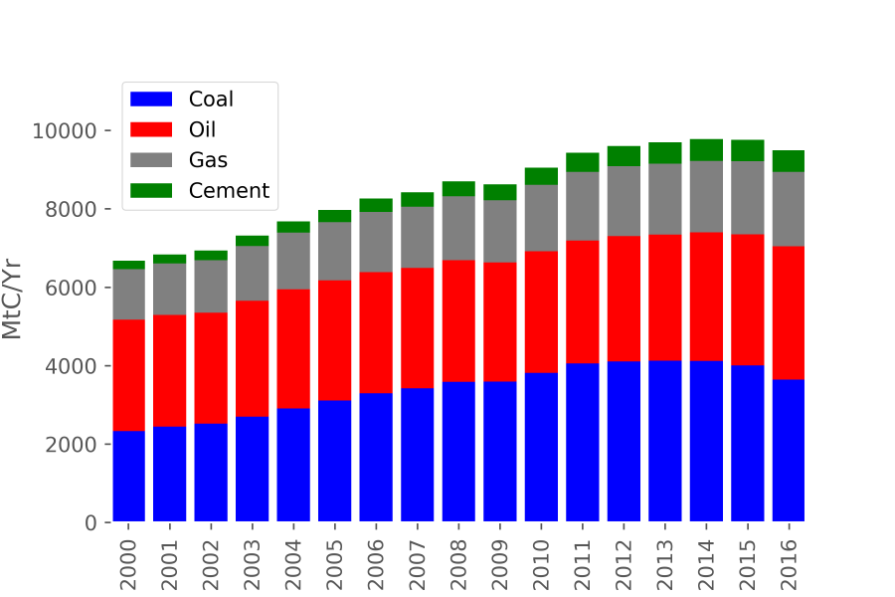
Greenhouse gas (GHG) emissions have brought about significant challenges, including, but not limited to, the rise of sea levels and devastating social effects. Carbon dioxide (CO2) is the most common GHG emitted by human activities, particularly the burning of fossil fuels (Figure 1). Different approaches to reduce CO2 accumulation in the atmosphere include using natural gas instead of coal, adopting renewable energies, and applying carbon capture and storage. Figure 2 shows that the demand for fossil fuels doubled between 2000 and 2016. This massive increase calls for an increased efforts in carbon capture.
Greenhouse gas (GHG) emissions have brought about significant challenges, including, but not limited to, the rise of sea levels and devastating social effects. Carbon dioxide (CO2) is the most common GHG emitted by human activities, particularly the burning of fossil fuels (Figure 1). Different approaches to reduce CO2 accumulation in the atmosphere include using natural gas instead of coal, adopting renewable energies, and applying carbon capture and storage. Figure 2 shows that the demand for fossil fuels doubled between 2000 and 2016. This massive increase calls for an increased efforts in carbon capture.
Technology based on adsorbents
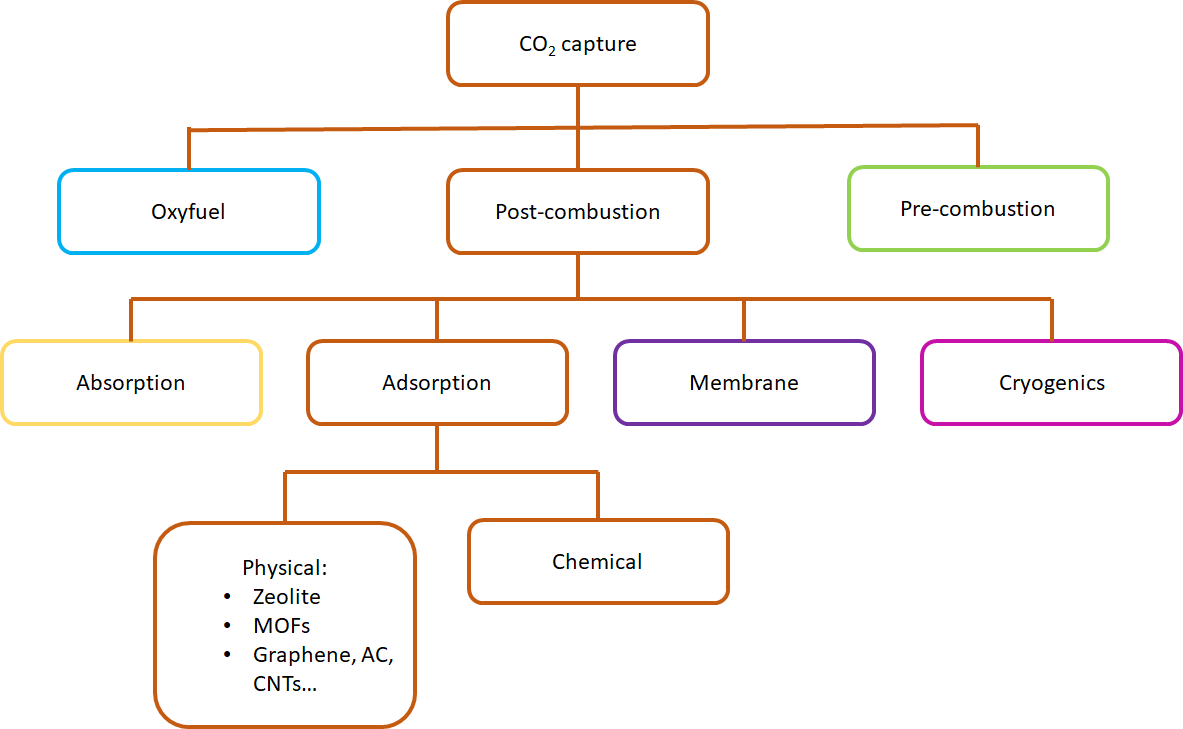
Although there are a myriad of techniques in the literature for carbon capture (Figure 3), physical adsorption is the most effective process, owing to its fast kinetics, cycling capability, and low regeneration energy. It is crucial to develop a material with a high potential CO2 adsorption and selectivity capacity. Porous materials are excellent candidates for physical adsorption because of their cost-effectiveness, high selectivity and high adsorption capacity (5). Among porous materials, hexagonal boron nitride (h-BN) has been a popular candidate for CO2 adsorption due to its unique properties, including large surface area, total pore volume, polarity, and various structural defects. Despite these features, CO2 adsorption on BN has been studied to a lesser extent than other technologies.
Although there are a myriad of techniques in the literature for carbon capture (Figure 3), physical adsorption is the most effective process, owing to its fast kinetics, cycling capability, and low regeneration energy. It is crucial to develop a material with a high potential CO2 adsorption and selectivity capacity. Porous materials are excellent candidates for physical adsorption because of their cost-effectiveness, high selectivity and high adsorption capacity (5). Among porous materials, hexagonal boron nitride (h-BN) has been a popular candidate for CO2 adsorption due to its unique properties, including large surface area, total pore volume, polarity, and various structural defects. Despite these features, CO2 adsorption on BN has been studied to a lesser extent than other technologies.
Synthesis and surface modification of porous boron nitride
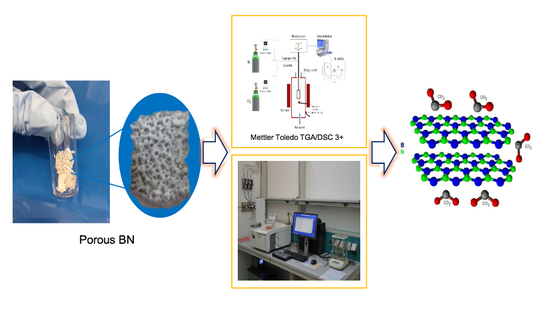
The porous structures of h-BN are synthesised via either template or non-template methods. The non-template method has relatively fewer steps as there is no need to remove the templates. Previous studies demonstrated that choice of synthesis method has significant effects on BN (7). One of the main targets for designing porous BNs is to achieve a material with a high surface area and porosity for CO2 adsorption. In view of this, a surface modification strategy was applied to tune BN, aiming to enhance the adsorption performance. The results of our previous study indicated that modified BN increased the interaction between BN and CO2 molecules by creating a higher level of porosity. The CO2 adsorption and desorption performance of the BN sample were evaluated by thermogravimetric analysis (Mettler Toledo TGA/DSC 3+). The capacity of the sorbents was determined by measuring the mass uptake of the sample during CO2 adsorption. The results showed that CO2 uptake on modified BN was enhanced by about 34.5% relative to pristine BN (2.69 mmol g-1 for BN-P123 vs. 2.00 mmol g-1 for pristine BN under ambient conditions) (8). Figure 4 offers a schematic representation of thermogravimetric analyser for capturing CO2 using porous BN.
The porous structures of h-BN are synthesised via either template or non-template methods. The non-template method has relatively fewer steps as there is no need to remove the templates. Previous studies demonstrated that choice of synthesis method has significant effects on BN (7). One of the main targets for designing porous BNs is to achieve a material with a high surface area and porosity for CO2 adsorption. In view of this, a surface modification strategy was applied to tune BN, aiming to enhance the adsorption performance. The results of our previous study indicated that modified BN increased the interaction between BN and CO2 molecules by creating a higher level of porosity. The CO2 adsorption and desorption performance of the BN sample were evaluated by thermogravimetric analysis (Mettler Toledo TGA/DSC 3+). The capacity of the sorbents was determined by measuring the mass uptake of the sample during CO2 adsorption. The results showed that CO2 uptake on modified BN was enhanced by about 34.5% relative to pristine BN (2.69 mmol g-1 for BN-P123 vs. 2.00 mmol g-1 for pristine BN under ambient conditions) (8). Figure 4 offers a schematic representation of thermogravimetric analyser for capturing CO2 using porous BN.
Conclusion
An overview of research on carbon capture technology and the potential of BN-based materials as solid adsorbents in CO2 capture has been presented in this report. Further study could investigate how to tailor the electronic properties of BN and introduce more active sites to increase CO2 capture. The principal characteristics of porous BN for CO2 adsorption (adsorption rate, adsorption capacity and ease of regeneration) offer other potential avenues of research.
An overview of research on carbon capture technology and the potential of BN-based materials as solid adsorbents in CO2 capture has been presented in this report. Further study could investigate how to tailor the electronic properties of BN and introduce more active sites to increase CO2 capture. The principal characteristics of porous BN for CO2 adsorption (adsorption rate, adsorption capacity and ease of regeneration) offer other potential avenues of research.
References
- [United Nations Climate Change, The Paris Agreement, (2016). [Online]. Available: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement. [Accessed: 16-Dec-2019].
- Webley, P.A., and Danaci, D. Chapter 5: CO2 Capture by Adsorption Processes, 26 (2020).
- Butler, J.H., and Montzka, S.A. THE NOAA ANNUAL GREENHOUSE GAS INDEX (AGGI), NOAA Earth System Research Laboratory, 2019. [Online]. Available: https://www.esrl.noaa.gov/gmd/aggi/aggi.html. [Accessed: 03-Sep-2019]
- Global Carbon Project (GCP), www.globalcarbonproject.org. [Accessed: 03-Sep-2017]
- Oschatz, M., and Antonietti, M., Energy & Environmental Science, 11, 1, 57–70 (2018)
- Younas, M., Sohail, M., Kong, L.L., Bashir, M.J.K., and Sethupathi, S. International Journal of Environmental Science & Technology, 13, 7, 1839–1860 (2016)
- Mishra, N.S., and Saravanan, P. A, Chemistry Select, 3, 28, 8023–8034 (2018).
- Hojatisaeidi, F., Mureddu, M., Dessì, F., Durand, G., and Saha, B., Energies, 13