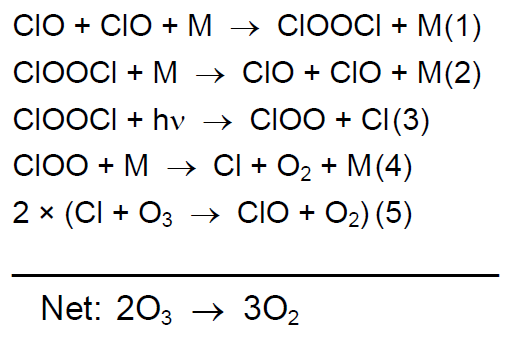Ozone hole paradigm is challenged by a new laboratory measurement of the chlorine peroxide spectrum
Data published in 2007 on the rate of photolysis of chlorine peroxide (Cl2O2) have cast doubts on the accepted mechanisms of ozone depletion in the stratosphere. Francis Pope compares the published kinetic analyses of the breakdown of Cl2O2 and comments on the significance of these measurements.
Introduction
Since 1985 when Farman and his colleagues first discovered the Antarctic ozone hole [1], great effort has been made to elucidate the mechanisms responsible for the loss of polar stratospheric ozone in both the Arctic and Antarctica.
Since 1985 when Farman and his colleagues first discovered the Antarctic ozone hole [1], great effort has been made to elucidate the mechanisms responsible for the loss of polar stratospheric ozone in both the Arctic and Antarctica.
|
The mechanism generally accepted to be largely responsible for polar ozone loss involves catalytic chlorine oxide (ClOx) species (Box 1). The cycle was first proposed by Molina and Molina and, according to the current consensus, accounts for around 60% of ozone loss [2].
Chlorine peroxide, ClOOCl, is a crucial intermediate in this cycle. High concentrations are built up during the cold and dark polar winter as an active chlorine reservoir. When spring arrives, solar radiation of suitable wavelengths reaches the stratosphere with which ClOOCl photolysis can occur, yielding two ozone destroying chlorine atoms. The photolysis rate of ClOOCl is critical to the efficiency of this scheme. The faster the ClOOCl photolysis, the greater is the fraction of ozone loss that can be attributed to the catalytic cycle shown in Box 1. |
Determination of ClOOCl photolysis rate
The photolysis rate is dependent on the wavelength integrated product of the ClOOCl absorption cross sections, the quantum yield of ClOOCl, and the flux of available light. Many measurements of the ClOOCl cross sections have been attempted previously. However, in the atmospherically important wavelength range, 300 – 500 nm, significant discrepancies exist between the different experiments. The ClOOCl molecule is difficult to generate, store and use. These factors make the determination of the absorption cross sections of ClOOCl very challenging. Additionally, synthesis must be performed in situ because the ClOOCl molecule is weakly bound and only stable at low stratospheric temperatures.
Several methods of ClOOCl synthesis have been described in the literature. Unfortunately, all synthetic routes result in the co-production of spectral impurities. Therefore, subtraction of the spectral impurities from the experimentally recorded spectrum must be performed to retrieve the true ClOOCl spectrum. The differences in the chosen methods of subtraction are probably the biggest cause of discrepancies between spectra in the literature. Many experimental data sets also suffer from poor signal to noise due to small concentrations of ClOOCl used in the measurements.
The recent work of Pope et al [3] generated much higher concentrations of ClOOCl than previously attained by other studies. Dramatic reductions in spectral impurities were also achieved using a novel step in the synthesis. Subsequent to generation, the ClOOCl was collected in a cold trap with its spectral impurities. After collection, gradual heating of the trap resulted in species leaving the trap at different times, depending on their volatility. Complete separation was not possible and spectral subtraction was still required, albeit at much lower levels than other work. The resulting Pope et al ClOOCl spectrum has absorption cross sections values significantly lower than other studies [3].
The photolysis rate is dependent on the wavelength integrated product of the ClOOCl absorption cross sections, the quantum yield of ClOOCl, and the flux of available light. Many measurements of the ClOOCl cross sections have been attempted previously. However, in the atmospherically important wavelength range, 300 – 500 nm, significant discrepancies exist between the different experiments. The ClOOCl molecule is difficult to generate, store and use. These factors make the determination of the absorption cross sections of ClOOCl very challenging. Additionally, synthesis must be performed in situ because the ClOOCl molecule is weakly bound and only stable at low stratospheric temperatures.
Several methods of ClOOCl synthesis have been described in the literature. Unfortunately, all synthetic routes result in the co-production of spectral impurities. Therefore, subtraction of the spectral impurities from the experimentally recorded spectrum must be performed to retrieve the true ClOOCl spectrum. The differences in the chosen methods of subtraction are probably the biggest cause of discrepancies between spectra in the literature. Many experimental data sets also suffer from poor signal to noise due to small concentrations of ClOOCl used in the measurements.
The recent work of Pope et al [3] generated much higher concentrations of ClOOCl than previously attained by other studies. Dramatic reductions in spectral impurities were also achieved using a novel step in the synthesis. Subsequent to generation, the ClOOCl was collected in a cold trap with its spectral impurities. After collection, gradual heating of the trap resulted in species leaving the trap at different times, depending on their volatility. Complete separation was not possible and spectral subtraction was still required, albeit at much lower levels than other work. The resulting Pope et al ClOOCl spectrum has absorption cross sections values significantly lower than other studies [3].
Atmospheric implications of the Pope et al data set
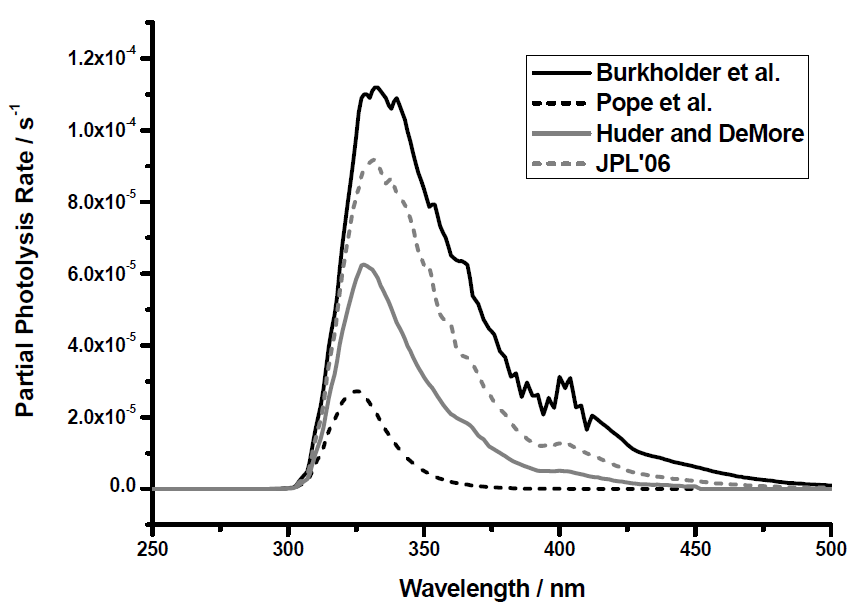
The Pope et al absorption cross sections [3] strongly affect the calculated ClOOCl photolysis rates. Figure 1 shows the partial photolysis rates calculated for a typical Arctic polar vortex for several different evaluations of the ClOOCl cross sections. Additional parameters used in the calculation where as follows: solar zenith angle of 86°, 20 km altitude, and typical O3 and temperature profiles. Figure 1 illustrates, visually and quantitatively, the difference between new and previously measured cross sections.
The Pope et al absorption cross sections [3] strongly affect the calculated ClOOCl photolysis rates. Figure 1 shows the partial photolysis rates calculated for a typical Arctic polar vortex for several different evaluations of the ClOOCl cross sections. Additional parameters used in the calculation where as follows: solar zenith angle of 86°, 20 km altitude, and typical O3 and temperature profiles. Figure 1 illustrates, visually and quantitatively, the difference between new and previously measured cross sections.
|
The integrated area under the curves represents the total photolysis rate. Total photolysis rates reported in references [4], [5] and [6] are respectively 8.9, 6.2, and 3.5 times larger than the Pope et al evaluation [3]. The Burkholder et al data set [4] is the largest valued data set in the literature. The data from Huder and DeMore [5] is used as the basis for the IUPAC recommendation . The Jet Propulsion Laboratory 2006 evaluation [6] is an average of various studies. The Burkholder et al data set [4] allows for the greatest agreement between modelled and measured O3 loss. Use of smaller cross sections reduces the modelled O3 loss.
The calculated ozone loss that results from the inclusion of the lower Pope et al absorption cross sections leads to a severe underestimation of the observed O3 loss. This underestimation is too large to allow for the possibility of adjustment of other model parameters. The result is also not model-specific, with the same outcome obtained from several different models [7]. If the Pope et al cross sections are correct, then the currently accepted chemical mechanisms of O3 loss are incomplete. This result is controversial and has led to perspective pieces in both Nature [8] (see p. 23 below) and Science [9]. |
Future work and open questions
Clearly the controversial data of Pope et al must now be verified or refuted, necessitating further experimental studies. The new experimental techniques developed in the Pope et al study eased some of the difficulties that marked previous studies. Is it possible to synthesise ClOOCl at higher purities than even those attained in the Pope et al study? If this does not prove feasible, then independent verification of spectral impurities, and in particular the troublesome Cl2, might yet be achieved. If the Pope et al absorption cross sections prove to be reproducible, then the hunt for the missing mechanism will begin in earnest. What are the possibilities for missing mechanisms? Reasonable candidates could be evaluated in atmospheric models before experimental work begins. It is likely that some species and schemes have already been considered, but might need to be revisited.
Clearly the controversial data of Pope et al must now be verified or refuted, necessitating further experimental studies. The new experimental techniques developed in the Pope et al study eased some of the difficulties that marked previous studies. Is it possible to synthesise ClOOCl at higher purities than even those attained in the Pope et al study? If this does not prove feasible, then independent verification of spectral impurities, and in particular the troublesome Cl2, might yet be achieved. If the Pope et al absorption cross sections prove to be reproducible, then the hunt for the missing mechanism will begin in earnest. What are the possibilities for missing mechanisms? Reasonable candidates could be evaluated in atmospheric models before experimental work begins. It is likely that some species and schemes have already been considered, but might need to be revisited.
Summary
The absorption cross sections of ClOOCl have been measured multiple times, with widely varying values in the atmospherically important wavelength window. New work by Pope et al generated purer and higher concentrations of ClOOCl than were previously attainable. Their measurements yielded controversially low values for the ClOOCl absorption cross sections. If correct, the new results necessitate rethinking the mechanism that generates the ozone hole. Future experimental work is now required so that consensus on the correct cross sections may be reached.
Acknowledgements
Many of the ideas mentioned in this topic have come about from discussions with the following people: Kyle Bayes, Tim Canty, Tony Cox, Marcus Rex, Stan Sander, Ross Salawitch, Fred Stroh, and Marc von Hobe.
Dr FRANCIS POPE,
Centre for Atmospheric Science,
Department of Chemistry,
University of Cambridge,
Cambridge, CB2 1EW
[email protected]
References
Biographical Note: After completing his PhD at Bristol University (2001-2004), Dr Francis Pope carried out the work described in this article as a Postdoctoral Scholar in Dr Sander’s group at the NASA Jet Propulsion Laboratory, California Institute of Technology during 2004 to 2006. Francis is currently working with Dr R. A. Cox at the Centre for Atmospheric Science, Department of Chemistry, University of Cambridge.
The absorption cross sections of ClOOCl have been measured multiple times, with widely varying values in the atmospherically important wavelength window. New work by Pope et al generated purer and higher concentrations of ClOOCl than were previously attainable. Their measurements yielded controversially low values for the ClOOCl absorption cross sections. If correct, the new results necessitate rethinking the mechanism that generates the ozone hole. Future experimental work is now required so that consensus on the correct cross sections may be reached.
Acknowledgements
Many of the ideas mentioned in this topic have come about from discussions with the following people: Kyle Bayes, Tim Canty, Tony Cox, Marcus Rex, Stan Sander, Ross Salawitch, Fred Stroh, and Marc von Hobe.
Dr FRANCIS POPE,
Centre for Atmospheric Science,
Department of Chemistry,
University of Cambridge,
Cambridge, CB2 1EW
[email protected]
References
- Farman, J. C., Gardiner, B. G., and Shanklin, J. D. (1985). Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature, 315, 207.
- Molina, L. T., and Molina, M. J. (1987). Production of chlorine oxide (Cl2O2) from the self-reaction of the chlorine oxide (ClO) radical. J. Phys. Chem., 91, 433.
- Pope, F .D., Hansen, J. C., Bayes, K. D., Friedl, R. R., and Sander, S. P. (2007). Ultraviolet absorption spectrum of chlorine peroxide, ClOOCl. J. Phys. Chem. A, 111, 4322.
- Burkholder, J. B., Orlando, J. J., Howard, C. J. (1990). Ultraviolet absorption cross sections of chlorine oxide (Cl2O2) between 210 and 410 nm. J. Phys. Chem., 94, 687.
- Huder, K. J., DeMore, W. B. (1995). Absorption cross sections of the ClO dimer. J. Phys. Chem., 99, 3905.
- Sander, S. P., Ravishankara, A. R., Golden, D. M., Kolb, C.E., Kurylo, M. J., Molina, M. J., Moortgat, G. K., Finlayson-Pitts, B. J., Wine, P. H., Huie, R. E., Orkin, V. L. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies: Evaluation Number 15. Jet Propulsion Laboratory: Pasadena, CA, 2006.
- von Hobe, M., Salawitch, R.J., Canty, T., Keller-Rudek, H., Moortgat, G.K., Grooß, J.-U., Müller, R., and Stroh, F. (2007). Understanding the kinetics of the ClO dimer cycle. Atmos. Chem. Phys., 7, 3055. [available free on the web]
- Schiermeier, Q. (2007). Chemists poke holes in ozone theory. Nature, 449, 382.
- von Hobe, M. (2007). Revisiting ozone depletion. Science, 318, (21st December), 1878.
Biographical Note: After completing his PhD at Bristol University (2001-2004), Dr Francis Pope carried out the work described in this article as a Postdoctoral Scholar in Dr Sander’s group at the NASA Jet Propulsion Laboratory, California Institute of Technology during 2004 to 2006. Francis is currently working with Dr R. A. Cox at the Centre for Atmospheric Science, Department of Chemistry, University of Cambridge.