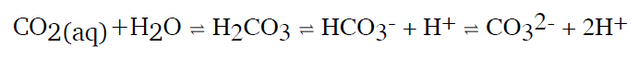Ocean acidification experiment
An interactive demonstration with colour change, bubbles and “smoke” that explains ocean acidification using dry ice as the CO2 source. Even adults are drawn to this old-fashioned, mad scientist, overflowing-flask demonstration. This exhibition guideline explains how to set up and introduce ocean acidification to the public.
Theory
Theory
Ocean acidification is linked to the increasing amount of CO2 that is present in the atmosphere (from fossil fuel combustion) dissolving in the oceans. There is a delicate balance between the carbon dioxide level in the atmosphere and the ocean, and the ocean is a sink for the excess CO2 in the atmosphere. The down-side of this process is the formation of H2CO3 (carbonic acid) which inhibits shell growth in marine animals.
Dissolving CO2 in seawater increases the hydrogen ion (H+) concentration in the ocean and thus decreases ocean pH, as follows:
Dissolving CO2 in seawater increases the hydrogen ion (H+) concentration in the ocean and thus decreases ocean pH, as follows:
See https://en.wikipedia.org/wiki/Ocean_acidification for further details.
Calcium carbonate minerals are the building blocks for the skeletons and shells of marine animals and ocean acidification can cause it to become undersaturated in these minerals. Corals are also very sensitive to pH (as well as temperature changes).
The ocean’s pH has decreased by 0.1 pH units since the industrial revolution and as the pH scale is logarithmic, this change represents a 30% increase in acidity.
Set up
Calcium carbonate minerals are the building blocks for the skeletons and shells of marine animals and ocean acidification can cause it to become undersaturated in these minerals. Corals are also very sensitive to pH (as well as temperature changes).
The ocean’s pH has decreased by 0.1 pH units since the industrial revolution and as the pH scale is logarithmic, this change represents a 30% increase in acidity.
Set up
Pour a small amount (100ml) of NaOH solution (see kit list for concentration) into the flask. Add sufficient indicator to produce a strong alkali colour. Add a few dry ice pellets and watch the solution bubble as sublimation (solid to gas) of CO2 gas occurs (it is very visible as the freezing temperatures condense the water, making it appear as a white, cold gas that escapes). The indicator will change to the acid colour during the reaction. A new solution needs to be prepared each time so keep a waste container nearby.
Challenge
What does the colour change tell us?
What does the colour change tell us?
|
Resources required
Dry ice (to be kept in an insulated box) Kit list • Round-bottomed flask (or large cylinder) • Universal Indicator solution • 1 litre 0.1 M NaOH solution • 2 litres water (to dilute NaOH:water, 1:2) • Dry ice pellets • pH table with scale for the indicator you use • Large container to pour excess solution from each experiment • Pictures of healthy coral and coral damage at lower pH |
Exhibition Cost
About £80 when using 2 bags of dry ice. Your nearest chemistry department could probably give you some for free. BOC delivers to the exhibit location but you may need to open a new account with any new delivery address. A 10kg bag costs around £30, but the delivery will be extra!
Exhibition weight
About 25kg
Exhibition size
About 1 cubic metre, depending on flask sizes
Things to look out for
Remember to be aware of the health and safety challenges of this experiment, and have trained staff handling the dry ice wearing insulated gloves.
About £80 when using 2 bags of dry ice. Your nearest chemistry department could probably give you some for free. BOC delivers to the exhibit location but you may need to open a new account with any new delivery address. A 10kg bag costs around £30, but the delivery will be extra!
Exhibition weight
About 25kg
Exhibition size
About 1 cubic metre, depending on flask sizes
Things to look out for
Remember to be aware of the health and safety challenges of this experiment, and have trained staff handling the dry ice wearing insulated gloves.
| ocean_acidification.pdf |