Natural analogues for nuclear waste: a window into the future?
Clare L. Thorpe, Claire L. Corkhill, Russell J. Hand
University of Sheffield
[email protected]
ECG Bulletin July 2021
University of Sheffield
[email protected]
ECG Bulletin July 2021
Glassy materials – those with no long-range atomic structure – are ubiquitous in our modern lives. Glass is formed when a molten liquid cools rapidly. Although many substances can be induced to form a glass, few do so as readily as silica (SiO2), making silicate glasses the most common. Other elements may substitute into the glass structure, either as covalent network formers (e.g. B and Al) in place of Si, or as network modifiers (e.g. Na+, K+, Ca2+, or Fe2+) that form ionic bonds with oxygen from the silicate network.
The addition of other elements to silicate glass has a variety of effects, such as lowering melting temperatures, improving physical or chemical durability, and changing the colour of the glass. The longevity of glass across a range of natural environments is of interest from the point of view of building construction, the manufacture of components such as semiconductors and fibre optics, the preservation of art and archaeological artefacts, and the safe disposal of vitrified waste. The impetus to understand glass degradation over very long time periods (> 10,000 years) primarily arises from the need to ensure that vitrified radioactive waste emplaced in subsurface disposal facilities will not pose a hazard to future generations.
In the UK, and in many countries including the USA, France, Russia, Germany and Japan, high level radioactive waste is formed from reprocessing spent nuclear fuel. This waste is immobilised by vitrification in a borosilicate glass matrix and destined for eventual disposal in an engineered geological facility. Vitrification is also being considered for lower radioactivity waste streams due to the wide range of elements that glass can incorporate, its chemical durability and resistance to radiation damage. Prior to geological disposal, the glass waste form is usually contained in a steel canister designed to keep the waste isolated from groundwater ingress for tens to hundreds of years, even after the surrounding backfill materials and geological units become saturated. However, radioactive waste needs to be isolated from the biosphere for thousands to tens of thousands of years (depending on the radioisotope in question) to allow the radioactivity to decay to safe levels. The rate of corrosion of the glass matrix is therefore an important factor in the safety case for disposal of vitrified radioactive wastes.
In the UK, and in many countries including the USA, France, Russia, Germany and Japan, high level radioactive waste is formed from reprocessing spent nuclear fuel. This waste is immobilised by vitrification in a borosilicate glass matrix and destined for eventual disposal in an engineered geological facility. Vitrification is also being considered for lower radioactivity waste streams due to the wide range of elements that glass can incorporate, its chemical durability and resistance to radiation damage. Prior to geological disposal, the glass waste form is usually contained in a steel canister designed to keep the waste isolated from groundwater ingress for tens to hundreds of years, even after the surrounding backfill materials and geological units become saturated. However, radioactive waste needs to be isolated from the biosphere for thousands to tens of thousands of years (depending on the radioisotope in question) to allow the radioactivity to decay to safe levels. The rate of corrosion of the glass matrix is therefore an important factor in the safety case for disposal of vitrified radioactive wastes.
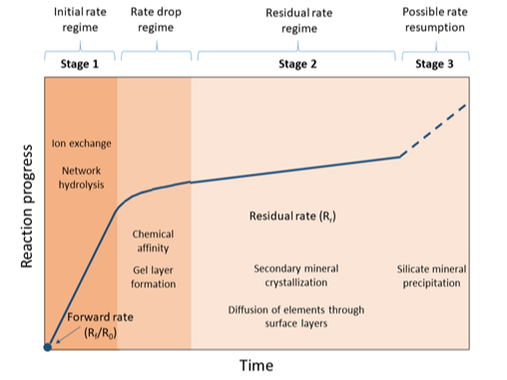
There is scientific consensus that glasses start to corrode when in contact with water or water vapour, and that the general mechanism begins with ion exchange between H+/H3O+ from the water and alkali metals (e.g. Li+, Na+, K+) from the glass. Concurrently, hydrolysis of the glass network leads to the breakdown of Si-O and B-O bonds (1). Elements released from the glass accumulate in solution until sufficient silica is freed to allow the formation of an amorphous gel layer on the glass surface. This layer densifies over time and can crystallise into secondary mineral phases (2), meaning that further corrosion of the bulk glass is limited by the diffusion through this surface alteration layer and the rate of release of elements from the glass decreases (Figure 1). The rate of glass dissolution can continue at this reduced rate indefinitely, or, under some conditions, the precipitation of certain minerals, for example zeolites, can drive an increase in the glass dissolution rate (3, 4).
|
Although the glass corrosion mechanism is well established, rates and timescales remain unpredictable, as the progress of glass degradation is dependent on a number of factors including pH, temperature, glass composition and solution chemistry. The rates of glass corrosion increase with increasing temperature and extreme pH conditions (either acidic or alkaline) can promote hydrolysis of the silicate network. The initial durability of the glass is highly dependent on its chemistry, with more polymerised networks – those with more ‘bridging’ oxygens – showing the greatest durability.
|
Additionally, both the solution chemistry and glass chemistry can greatly influence the composition of the gel layer formation and secondary mineral precipitates.
To date, most estimates of glass corrosion rates have been derived from accelerated laboratory tests. In order to achieve measurable results over a short time period, laboratory tests are accelerated by increasing the glass surface area and raising the temperature, often to ≥ 90 °C. In addition, processes that occur only in complex natural environments and are not observable in simplified, sterile laboratory tests may influence corrosion. These include the direct and indirect effect of microbial metabolism, the effect of changing or fluctuating geochemistry, and variable saturation which are, as yet, poorly understood.
Natural and archaeological analogues
One way to validate the results obtained from static laboratory testing is by comparison with natural and ancient glasses that have been exposed to a known environment for a known period. These samples have come to be termed ‘natural analogues’; their behaviour provides an analogue for that of modern nuclear waste glasses that have only been manufactured in the last 50 years.
Natural glasses include igneous glasses of basaltic (rich in Fe and Mg) and rhyolitic (rich in Si) compositions, fulgurites (petrified lightning strikes), glass observed in meteorites and impact glasses (Figure 2).
To date, most estimates of glass corrosion rates have been derived from accelerated laboratory tests. In order to achieve measurable results over a short time period, laboratory tests are accelerated by increasing the glass surface area and raising the temperature, often to ≥ 90 °C. In addition, processes that occur only in complex natural environments and are not observable in simplified, sterile laboratory tests may influence corrosion. These include the direct and indirect effect of microbial metabolism, the effect of changing or fluctuating geochemistry, and variable saturation which are, as yet, poorly understood.
Natural and archaeological analogues
One way to validate the results obtained from static laboratory testing is by comparison with natural and ancient glasses that have been exposed to a known environment for a known period. These samples have come to be termed ‘natural analogues’; their behaviour provides an analogue for that of modern nuclear waste glasses that have only been manufactured in the last 50 years.
Natural glasses include igneous glasses of basaltic (rich in Fe and Mg) and rhyolitic (rich in Si) compositions, fulgurites (petrified lightning strikes), glass observed in meteorites and impact glasses (Figure 2).
Ancient glass samples include those found at Roman and Medieval burial sites, the walls of vitrified Hill Forts, and those retrieved from shipwrecks where the start date of their contact with water or soil can be well constrained (Figure 3). These sources provide a surprising range of glass compositions from soda-lime and lead silicates used to make bottles and decorative artefacts to metal-rich vitreous slags formed as by-products from copper smelting.
The major limitation when comparing the dissolution of natural or ancient glasses with that of nuclear waste glasses lies in compositional differences and, notably, the absence of boron. Boron oxide (B2O3) is included in most nuclear waste glass composition at 5-25 weight %, where it substitutes into the silicate network and improves chemical durability. As borosilicate glass was only commercially manufactured from the early 1900s and nuclear waste compositions only from the 1970s, there are no older samples available to study. Therefore, purposeful long term burial experiments have been established under a variety of conditions relevant to geological waste disposal, including in different geological lithologies (granite, salt, limestone and clay) (5-8). Sometimes referred to as ‘field tests’, these studies aim to bridge the gap between precise, short term, laboratory studies and observations from natural analogue studies where differences in glass composition and exact environmental conditions may lead to uncertainty.
UK example studies
The University of Sheffield Immobilisation Science Laboratory specialises in developing new thermal treatment solutions for radioactive wastes and in assessing the long term durability of both new and existing vitreous waste forms. A number of natural analogue studies are underway to complement laboratory-based dissolution testing and investigate glass corrosion in complex natural environments.
Ballidon long-duration experiment
The Ballidon Quarry, Derbyshire, UK, hosts one of the longest running glass burial experiments in the world (Figure 4). Now in its 50th year, this site was originally established to test the degradation of archaeological glasses under alkaline conditions compared to a sister experiment in acidic soil (9). Later, simulant (non-radioactive) nuclear waste glasses were added to the experiment, along with climate monitoring equipment to ensure conditions at Ballidon are well constrained before the next set of samples are removed in 2022.
The major limitation when comparing the dissolution of natural or ancient glasses with that of nuclear waste glasses lies in compositional differences and, notably, the absence of boron. Boron oxide (B2O3) is included in most nuclear waste glass composition at 5-25 weight %, where it substitutes into the silicate network and improves chemical durability. As borosilicate glass was only commercially manufactured from the early 1900s and nuclear waste compositions only from the 1970s, there are no older samples available to study. Therefore, purposeful long term burial experiments have been established under a variety of conditions relevant to geological waste disposal, including in different geological lithologies (granite, salt, limestone and clay) (5-8). Sometimes referred to as ‘field tests’, these studies aim to bridge the gap between precise, short term, laboratory studies and observations from natural analogue studies where differences in glass composition and exact environmental conditions may lead to uncertainty.
UK example studies
The University of Sheffield Immobilisation Science Laboratory specialises in developing new thermal treatment solutions for radioactive wastes and in assessing the long term durability of both new and existing vitreous waste forms. A number of natural analogue studies are underway to complement laboratory-based dissolution testing and investigate glass corrosion in complex natural environments.
Ballidon long-duration experiment
The Ballidon Quarry, Derbyshire, UK, hosts one of the longest running glass burial experiments in the world (Figure 4). Now in its 50th year, this site was originally established to test the degradation of archaeological glasses under alkaline conditions compared to a sister experiment in acidic soil (9). Later, simulant (non-radioactive) nuclear waste glasses were added to the experiment, along with climate monitoring equipment to ensure conditions at Ballidon are well constrained before the next set of samples are removed in 2022.
Peak Dale Cave
In Peak Dale, Derbyshire, UK, a man-made tunnel beneath a lime workings provides a stable environment with hyperalkaline, Ca-rich water chemistry analogous to the cement leachates expected to evolve over time within a geological disposal facility where a cementitious engineered barrier is used. In addition to analysing glass samples found in the cave estimated at > 70 years old (the date the tunnel was closed) (10, 11), researchers at the University of Sheffield have established a new field test including nuclear waste glass compositions from the UK and USA set to run for 30 years (Figure 5).
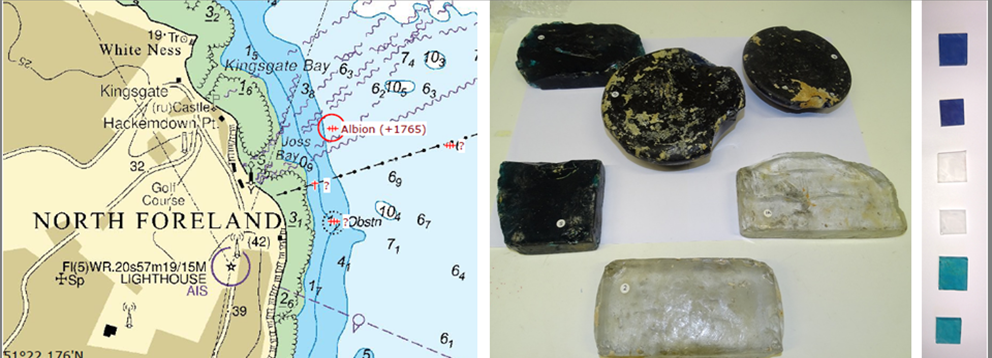
Albion shipwreck glasses
Analysis of 265 year old unique glass ingots (Figure 6), from a marine environment allows a low temperature corrosion comparison. Samples show evidence of biological colonisation and provide an opportunity to assess the effect of minor metal constituents of the glass (e.g. Cu, Co, and Mn), which were added as colourants but may also have biocidal effects. This work is funded by the Royal Society of Chemistry Research Fund.
In Peak Dale, Derbyshire, UK, a man-made tunnel beneath a lime workings provides a stable environment with hyperalkaline, Ca-rich water chemistry analogous to the cement leachates expected to evolve over time within a geological disposal facility where a cementitious engineered barrier is used. In addition to analysing glass samples found in the cave estimated at > 70 years old (the date the tunnel was closed) (10, 11), researchers at the University of Sheffield have established a new field test including nuclear waste glass compositions from the UK and USA set to run for 30 years (Figure 5).
Albion shipwreck glasses
Analysis of 265 year old unique glass ingots (Figure 6), from a marine environment allows a low temperature corrosion comparison. Samples show evidence of biological colonisation and provide an opportunity to assess the effect of minor metal constituents of the glass (e.g. Cu, Co, and Mn), which were added as colourants but may also have biocidal effects. This work is funded by the Royal Society of Chemistry Research Fund.
Black Bridge slag samples
Archaeological vitreous slag samples found within the town of Hayle, West Cornwall, may provide a close natural analogue to some heterogeneous glasses produced from mixed wastes such as plutonium contaminated materials (Figure 7) (12, 13). The Copperhouse foundry, first founded in 1758, and the Black Bridge, constructed circa 1811, can be used to date Hayle slag samples as ~ 250 years old. As the slag blocks were fixed in position, their exposure environment is known: depending on location, they were exposed to saline water, brackish water, or rainwater. A new study plans to investigate the corrosion of these materials and slags from other locations as an analogue for iron rich nuclear waste glasses.
Conclusions
No single natural analogue or field test site can tell us everything we need to know about the long term behaviour of glass. However, using a combination of many can increase our understanding of corrosion mechanisms under a wide variety of environments and decrease the margin of uncertainty around elemental release from vitrified radioactive wastes.
References
Archaeological vitreous slag samples found within the town of Hayle, West Cornwall, may provide a close natural analogue to some heterogeneous glasses produced from mixed wastes such as plutonium contaminated materials (Figure 7) (12, 13). The Copperhouse foundry, first founded in 1758, and the Black Bridge, constructed circa 1811, can be used to date Hayle slag samples as ~ 250 years old. As the slag blocks were fixed in position, their exposure environment is known: depending on location, they were exposed to saline water, brackish water, or rainwater. A new study plans to investigate the corrosion of these materials and slags from other locations as an analogue for iron rich nuclear waste glasses.
Conclusions
No single natural analogue or field test site can tell us everything we need to know about the long term behaviour of glass. However, using a combination of many can increase our understanding of corrosion mechanisms under a wide variety of environments and decrease the margin of uncertainty around elemental release from vitrified radioactive wastes.
References
- S. Gin, et al. (2013). New Insight into the Residual Rate of Borosilicate Glasses: Effect of S/V and Glass Composition. Int. J. Appl. Glass Sci., 4 (4) 371–382.
- S. Gin, et al. (2015a). Origin and consequences of silicate glass passivation by surface layers. Nat. Commun, 6, 8.
- M. Fournier, et al. (2017). Contribution of zeolite-seeded experiments to the understanding of resumption of glass alteration. Mater Degrad 1, 17.
- J. J. Neeway, et al. (2020) Acceleration of glass alteration rates induced by zeolite seeds at controlled pH. Appl. Geochem. 113, 104515.
- G. G. Wicks, (2001). US field testing programs and results. J. Nucl. Mater., 298, 1–2, 78-85.
- P. Van Iseghem, E. Valcke, and A. Lodding. (2001). In situ testing of the chemical durability of vitrified high-level waste in a Boom Clay formation in Belgium: discussion of recent data and concept of a new test. J. Nucl. Mater., 298, 1–2, 86-94.
- C. M. Jantzen et al. (2008). Performance of a buried radioactive high level waste (HLW) glass after 24 years. J. Nucl. Mater., 378, 3, 244-256.
- D. Bacon et al., (2018) Field-Scale Lysimeter Studies of Low-Activity Waste Form Degradation. Pacific Northwest National Laboratory, Richland, WA.
- W. W. Fletcher. (1972). The chemical durability of glass. A burial experiment at Ballidon in Derbyshire. J. Glass Stud., 14, 149-151.
- C. Mann, et al. (2017). Interactions between Simulant Vitrified Nuclear Wastes and high pH solutions: A Natural Analogue Approach. MRS Advances, 2 (12), 669–674.
- C. Mann et al. (2018). Dissolution of glass in cementitious solutions: An analogue study for vitrified waste disposal. MRS Advances, 3 (21), 1147–1154.
- N.C. Hyatt, et al. (2014). Thermal treatment of simulant plutonium contaminated materials from the Sellafield site by vitrification in a blast-furnace slag. J. Nucl. Mater., 444, 1–3, 186-199.
- A. Michelin et al. (2015). Archeological slag from Glinet: An example of silicate glass altered in an anoxic iron-rich environment. Chem. Geol., 413, 28-43.