Accessing powerful nanostructures of exfoliated biochar using green solvents
2021 #RSCPoster Twitter Conference, 2 March 2021 - 3 March 2021
Juliana Vidal
Memorial University of Newfoundland
[email protected]
ECG Bulletin July 2021
Juliana Vidal
Memorial University of Newfoundland
[email protected]
ECG Bulletin July 2021
By the 90s, environmental concerns led to the formulation of the principles of Green Chemistry, which involve the design and/or modification of chemical processes and products to minimise (or ideally eliminate) the use and generation of hazardous substances (1,2). Green Chemistry can help in the achievement of the UN’s 2015 Sustainable Development Goals (SDGs) and has become a profitable strategy for preserving the future of our planet (3). Yet how can we develop green products that are also successful in the marketplace?
|
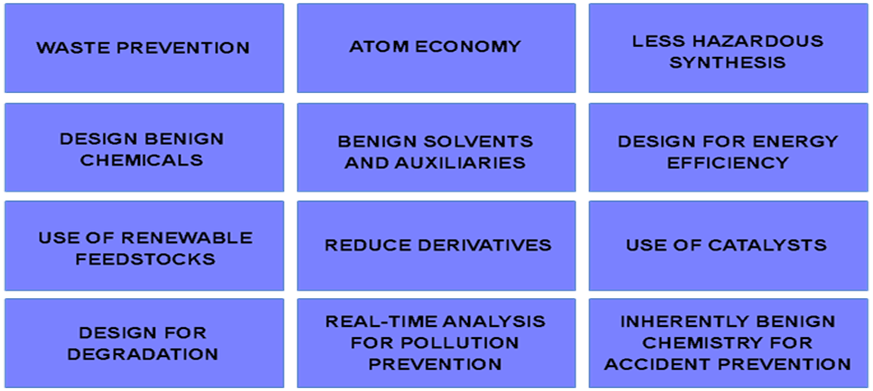
To solve this challenge, John Warner and Paul Anastas created, in 1998, the 12 Principles of Green Chemistry (Figure 1), a holistic guiding framework for chemistry to provide benefits to our society without harming human health and the environment (2).
Biochar and sustainable development Large amounts of wood waste are generated by paper mill industries and sawmills. The majority of the sludge, bark, and sawdust produced are transported to landfills or abandoned on the harvested sites, liberating greenhouse gases to our atmosphere during decomposition. |
If we use pyrolysis to transform the biomass waste into biochar instead of leaving it aside to decompose, the environmental problems of their decay can be mitigated while producing useful materials and energy (4).
Pyrolysis is the thermochemical decomposition of organic matter in an inert environment (i.e. in the absence of oxygen) in the temperature range typically 400–800°C. Through this treatment, waste biomass is transformed into biochar, a material that decomposes more slowly than wood or other forms of biomass (5). This process has the potential to mitigate carbon dioxide (CO2) emissions and climate change by rerouting carbon from a rapid biological cycle to a much slower cycle (6).
Pyrolysis is the thermochemical decomposition of organic matter in an inert environment (i.e. in the absence of oxygen) in the temperature range typically 400–800°C. Through this treatment, waste biomass is transformed into biochar, a material that decomposes more slowly than wood or other forms of biomass (5). This process has the potential to mitigate carbon dioxide (CO2) emissions and climate change by rerouting carbon from a rapid biological cycle to a much slower cycle (6).
|
The role of biochar as a carbon sink has been recognised by the Intergovernmental Panel on Climate Change (IPCC) in their 2014 report (7). Bio-oil and syngas are also produced during pyrolysis, and can be used as sources of renewable energy.
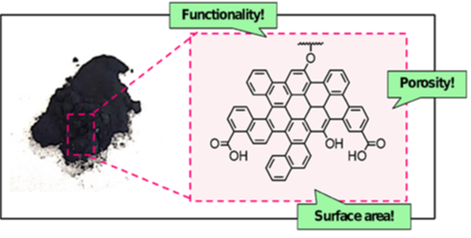
Biochar is a carbon material of high porosity, surface area, and surface functionality (Figure 2) (8). It is mainly used to remove pollutants from aqueous solutions and improve soil fertility. An increase in the utilisation of biochar in higher value-added fields not only represents an important approach in terms of climate change mitigation, but can also help to promote economic growth whilst reducing impacts of consumption. |
Making exfoliated biochar greener
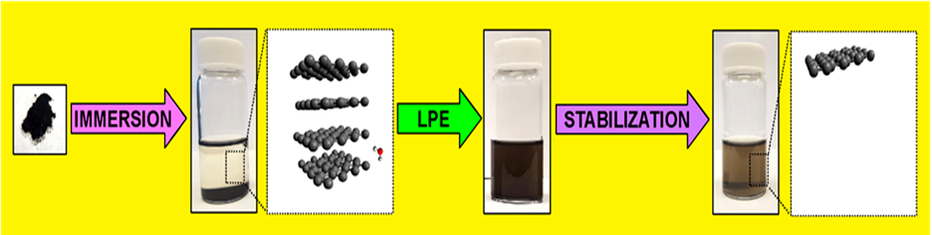
Layered materials are solids with strong in-plane bonds and weak interactions between layers. Through a process known as exfoliation, those weak interlayer attractions may be broken using external force, thus producing a single or a small number (< 10) of stacked nanosheets (9). When the material is immersed in a solvent and ultrasound applied as an external force, the process is then called Liquid-Phase Exfoliation (LPE) (Figure 3) (10). Biochar nanostructures (nanobiochar) obtained by LPE of biochar possess enhanced physico-chemical characteristics when compared to layered counterparts. Nanobiochar incorporated into other materials increases the commercial value of these products (11). The LPE of biochar and other carbon materials has been described in the literature (12-15). Solvent parameters such as surface tension and density play an essential role in the process (16). For example, if solvents with the appropriate properties are used, LPE is more efficient and requires less energy. The most commonly used solvent for LPE is N-methylpyrrolidone (NMP); an expensive, high-boiling point (202 °C), and toxic solvent. The mechanisms underpinning solvent selection for the LPE of biochar remains unclear. Our objective was to understand better these mechanisms and to identify benign alternative (green) solvents for the production of exfoliated biochar (17). Besides surface tension and density, we discovered that another set of solvent properties, known as Kamlet-Taft solvatochromic parameters, were responsible for facilitating the process. Depending on the hydrogen-bonding ability and polarisability of the solvent, samples could efficiently exfoliate biochar and its oxidised analogue from different biomass feedstocks (i.e. from hardwood and softwood trees). Moreover, biochar nanostructure samples containing between 2-8 layers (∼ 15 nm thickness) could be produced in greener alternative solvents than NMP with similar yields. These alternative solvents include dimethyl carbonate, ethyl acetate, polyethylene glycols, and e-caprolactone.
Layered materials are solids with strong in-plane bonds and weak interactions between layers. Through a process known as exfoliation, those weak interlayer attractions may be broken using external force, thus producing a single or a small number (< 10) of stacked nanosheets (9). When the material is immersed in a solvent and ultrasound applied as an external force, the process is then called Liquid-Phase Exfoliation (LPE) (Figure 3) (10). Biochar nanostructures (nanobiochar) obtained by LPE of biochar possess enhanced physico-chemical characteristics when compared to layered counterparts. Nanobiochar incorporated into other materials increases the commercial value of these products (11). The LPE of biochar and other carbon materials has been described in the literature (12-15). Solvent parameters such as surface tension and density play an essential role in the process (16). For example, if solvents with the appropriate properties are used, LPE is more efficient and requires less energy. The most commonly used solvent for LPE is N-methylpyrrolidone (NMP); an expensive, high-boiling point (202 °C), and toxic solvent. The mechanisms underpinning solvent selection for the LPE of biochar remains unclear. Our objective was to understand better these mechanisms and to identify benign alternative (green) solvents for the production of exfoliated biochar (17). Besides surface tension and density, we discovered that another set of solvent properties, known as Kamlet-Taft solvatochromic parameters, were responsible for facilitating the process. Depending on the hydrogen-bonding ability and polarisability of the solvent, samples could efficiently exfoliate biochar and its oxidised analogue from different biomass feedstocks (i.e. from hardwood and softwood trees). Moreover, biochar nanostructure samples containing between 2-8 layers (∼ 15 nm thickness) could be produced in greener alternative solvents than NMP with similar yields. These alternative solvents include dimethyl carbonate, ethyl acetate, polyethylene glycols, and e-caprolactone.
Conclusions
Nanobiochar can be used to improve the physical and mechanical properties of composite materials. The biochar nanostructures described in this study have potential use as fillers in the manufacture of cheaper biodegradable, non-fossil fuel derived, biocomposites.
We hope the work described here will encourage others to evaluate different solvents and mechanisms for the LPE of layered materials – thus developing the application of biochar and helping to implement the Twelve Principles of Green Chemistry.
References
The #RSCPoster Twitter Conference is an annual online event to bring the research community together over just 24 hours. In 2021, c 900 submissions were received from 62 countries, with 5,500 people discussing them on Twitter. Juliana Vidal was the RSC poster winner.
Nanobiochar can be used to improve the physical and mechanical properties of composite materials. The biochar nanostructures described in this study have potential use as fillers in the manufacture of cheaper biodegradable, non-fossil fuel derived, biocomposites.
We hope the work described here will encourage others to evaluate different solvents and mechanisms for the LPE of layered materials – thus developing the application of biochar and helping to implement the Twelve Principles of Green Chemistry.
References
- Lancaster, M., Green Chemistry: An Introductory Text. 3rd ed.; RSC Publishing: Cambridge, UK, 2016.
- Anastas, P. T.; Warner, J. C., Green Chemistry: Theory and Practice. Oxford University Press: 1998.
- Anastas, P. T.; Zimmerman, J. B., Curr. Opin. Green Sustain. Chem. 2018, 13, 150.
- Woolf, D.; Amonette, J. E.; Street-Perrott, F. A.; Lehmann, J.; Joseph, S., Nat. Commun. 2010, 1, 56.
- Wang, L.; O’Connor, D.; Rinklebe, J.; Ok, Y. S.; Tsang, D. C. W.; Shen, Z.; Hou, D., Environ. Sci. Technol. 2020, 54, 14797.
- Lehmann, J., Nature 2007, 447, 143.
- IPCC Fifth Assessment Report: Climate Change 2014 (AR5).
- Liu, W. J.; Jiang, H.; Yu, H. Q., Chem. Rev. 2015, 115, 12251.
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M. G.; Strano, M. S.; Coleman, J. N., Science 2013, 340, 1420.
- Backes, C.; Higgins, T. M.; Kelly, A.; Boland, C.; Harvey, A.; Hanlon, D.; Coleman, J. N., Chem. Mater. 2017, 29, 243.
- Tao, H.; Zhang, Y.; Gao, Y.; Sun, Z.; Yan, C.; Texter, J., Phys. Chem. Chem. Phys. 2017, 19, 921.
- Xiao, X.; Chen, B., Environ. Sci. Technol. 2017, 51, 5473.
- Liu, G.; Zheng, H.; Jiang, Z.; Zhao, J.; Wang, Z.; Pan, B.; Xing, B., Environ. Sci. Technol. 2018, 52, 10369.
- Oleszczuk, P.; Ćwikła-Bundyra, W.; Bogusz, A.; Skwarek, E.; Ok, Y. S., J. Anal. Appl. Pyrol. 2016, 121, 165.
- Tian, W.; Gao, Q.; VahidMohammadi, A.; Dang, J.; Li, Z.; Liang, X.; Hamedi, M. M.; Zhang, L., Chem. Eng. J. 2020, 127601.
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F. M.; Sun, Z.; De, S.; McGovern, I. T.; Holland, B.; Byrne, M.; Gun'Ko, Y. K.; Boland, J. J.; Niraj, P.; Duesberg, G.; Krishnamurthy, S.; Goodhue, R.; Hutchison, J.; Scardaci, V.; Ferrari, A. C.; Coleman, J. N., Nat. Nanotechnol. 2008, 3, 563.
- Vidal, J. L.; Gallant, S. M. V.; Connors, E. P.; Richards, D. D.; MacQuarrie, S. L.; Kerton, F. M., ChemRvix. Preprint 2020.
The #RSCPoster Twitter Conference is an annual online event to bring the research community together over just 24 hours. In 2021, c 900 submissions were received from 62 countries, with 5,500 people discussing them on Twitter. Juliana Vidal was the RSC poster winner.