Health concerns of metals and metalloids
There is a long history and an overwhelming amount of data on the toxicity of heavy metal compounds. Here we take a brief look at some aspects of the toxicity of arsenic, mercury, lead and cadmium, and highlight the contrast between the acute and chronic toxicity of purely inorganic metal and metalloid species and their organometallic derivatives.
|
Acute and chronic toxicity
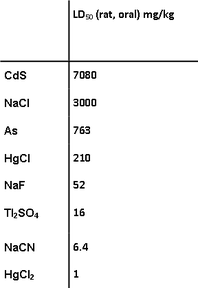
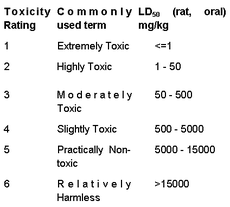
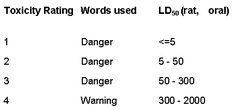
Sometimes the distinction between acute toxicity and chronic toxicity is not very clear, but generally acute toxicity is the result of a single exposure leading to illness or death, whereas chronic toxicity is the result of long-term exposure leading to illness or death. A measure of acute toxicity was originally developed to test the strength of pharmaceutical preparations which could not be measured by chemical means, for example, digitalis. Fox glove preparations have been used medically since the 18th century, sometimes with dire consequences because the concentration of the active principle, digitalis, depends on the quality of the plant and the efficacy of the extraction process. The difference between the dose required for a medical effect and that which could prove fatal is not large. A measure of the acute toxicity of pharmaceutical preparations was devised by Dr. John William Trevan (1887-1956) a pharmacologist who was director of the Wellcome Research Laboratories until he retired in 1953. In this test, a number of rats were given various single doses of the test material and from the number who died within a day or two a figure of the median lethal dose, LD50, could be determined (1). Typical values for LD50 are given in Table 1. It may be a surprise that cadmium sulfide is less toxic than sodium chloride, but that is only due to its low solubility; more noteworthy is that mercury(I) compounds are two hundred times less toxic than mercury(II) compounds. Some idea of the human toxicity of the compounds in the list can be can be gained by multiplying the LD50 value by the weight of a standard adult male, 60 kg in the EU or 80 kg in the USA. For many years it was customary to convert the numbers into words, using the Hodge and Sterner Scale (1949), which could then be used on labels (Table 2) (2). This has been replaced, in Europe, by the Globally Harmonised System of Classification and Labelling and Packaging of Chemicals, which is much simpler (Table 3) (3). |
Arsenic
Arsenic has been known from antiquity to be toxic and is popularly associated with accidental or deliberate poisoning leading to death. It was widely available until the mid-20th century and was used as a pesticide against rats but is now replaced by thallium salts or Warfarin. The use of arsenic, as the oxide, in taxidermy to preserve specimens from insect attack was introduced by Jean-Baptiste Bécoeur (1718-1777) and was in use in museums until the 1980s (4). A related use of arsenic compounds is as a herbicide: Agent Blue was one of the rainbow herbicides used against Vietnam to destroy rice paddies (5). Agent Blue is a mixture of cacodylic acid (dimethylarsinic acid) and its sodium salt. There was a significant use of coloured arsenic compounds by artists in painting (orpiment, As2S3, realgar, As4S4) and in wallpaper (Scheele’s green, copper arsenite, CuHAsO3 and Schweinfurt (or Schweinfurth) green, also termed Paris green, Vienna green, or emerald green (copper acetoarsenite, 3CuO·As2O3·Cu[OOC·CH3]).
Arsenic has been known from antiquity to be toxic and is popularly associated with accidental or deliberate poisoning leading to death. It was widely available until the mid-20th century and was used as a pesticide against rats but is now replaced by thallium salts or Warfarin. The use of arsenic, as the oxide, in taxidermy to preserve specimens from insect attack was introduced by Jean-Baptiste Bécoeur (1718-1777) and was in use in museums until the 1980s (4). A related use of arsenic compounds is as a herbicide: Agent Blue was one of the rainbow herbicides used against Vietnam to destroy rice paddies (5). Agent Blue is a mixture of cacodylic acid (dimethylarsinic acid) and its sodium salt. There was a significant use of coloured arsenic compounds by artists in painting (orpiment, As2S3, realgar, As4S4) and in wallpaper (Scheele’s green, copper arsenite, CuHAsO3 and Schweinfurt (or Schweinfurth) green, also termed Paris green, Vienna green, or emerald green (copper acetoarsenite, 3CuO·As2O3·Cu[OOC·CH3]).
The concern about the extent of accidental or deliberate poisoning in the 19th century led to the passing of the Sale of Arsenic Regulation Act (1851) which required a signature for the purchase of arsenic compounds (6). It was largely ineffective and poisoning continued over the next century. Arsenic acid was employed in the manufacture of the second aniline dye, fuchsine or magenta, and this was used to colour foodstuff like sausages or wine, but the dye could still contain enough residual arsenic to prove fatal. Manufacturers persisted in using arsenic acid as the oxidant, which “has led to many lamentable accidents”, leading to pleas in 1875 in Chemical News to discontinue (7).
An inspection of the LD50 values for a variety of arsenic compounds reveals several interesting aspects. First, arsine, AsH3, is highly toxic, as are methyl- and dimethyl-arsines, but trimethylarsine is practically non-toxic (8). Secondly, arsenite is more toxic than arsenate, but methylation reduces the toxicity to practically zero. There are two different mechanisms occurring here: (a) inorganic arsenic poisoning kills by allosteric inhibition of essential metabolic enzymes, leading to death from multi-system organ failure and methylation removes this pathway. And (b) arsine and methylated derivatives, but not trimethylarsine, generate methylarsinyl peroxyl radicals which destroy DNA (9). There is no doubt that dimethylarsine is highly reactive to air – it spontaneously ignites in air, burning with an orange flame, and generating a white smoke which has surprising sternutatory properties (10). Another characteristic property of volatile organoarsenic compounds is their intensely unpleasant smell. The odour threshold for trimethylarsine in dilute aqueous solution is found to be 2 ng kg-1 leading to the erroneous conclusion that anything so incredibly smelly must be toxic (11). Which neatly leads us to the question of wallpaper.
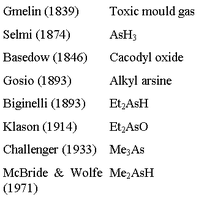
In the early 19th century, arsenical green pigments were used to colour wallpaper. It was soon noticed that when mouldy and damp, powerful arsenical odours were generated. This was supposed to be the source of famous poisoning incidents, e.g. Napoleon (12). The identity of the volatile arsine species was investigated at length by:
Attribution of poisoning due to the evolution of volatile arsenic compounds produced by arsenical compounds in wallpaper is now discredited (13), but in any case, the amounts generated would be very small, enough to cause ill-health but not death.
The chronic toxicity of inorganic arsenic compounds leading to skin cancers was first noted by Sir Jonathan Hutchinson (1828-1913) in 188714 and has been extensively studied (15). Of current concerns are the chronic health effects of arsenic-contaminated drinking water. The most significant regions affected are in S.E. Asia, in particular West Bengal, and Bangladesh where more than ten million people are at risk. Described by the World Health Organization as “the largest mass poisoning in history”, drinking water in these regions commonly contains 0.05-0.2 mg L–1 arsenic. A limit of <0.01 mg L–1 of arsenic in drinking water is the current WHO recommendation (16).
Mercury
Mercury was known in ancient times and the metal and its compounds have found diverse uses ever since. In earlier times, it was an essential component of the alchemists’ kit and major uses were in silver and gold mining, and in pharmaceutical preparations. More recently, industrial uses such as in the Castner-Kellner cells, in scientific measuring instruments for measuring temperature and pressure and the use in dental amalgams have increased the amount of mercury available in the environment. Other industrial activities, such as coal fired power plants make their contribution too. A natural source of mercury is volcanos. This is dramatically demonstrated from the results of analysis of the ice cores of the Fremont glacier in Wyoming (17). The pre-industrial level of mercury concentration is about 4 ng L-1 then in 1815 there is a spike corresponds to the eruption of a volcano at Tambora in Indonesia, which caused world-wide crop failures. The increase for the years 1850-1875 corresponds to the gold rush and the rise after World War I to the growth in industrial use which declined sharply after about 1985.
Mercury was known in ancient times and the metal and its compounds have found diverse uses ever since. In earlier times, it was an essential component of the alchemists’ kit and major uses were in silver and gold mining, and in pharmaceutical preparations. More recently, industrial uses such as in the Castner-Kellner cells, in scientific measuring instruments for measuring temperature and pressure and the use in dental amalgams have increased the amount of mercury available in the environment. Other industrial activities, such as coal fired power plants make their contribution too. A natural source of mercury is volcanos. This is dramatically demonstrated from the results of analysis of the ice cores of the Fremont glacier in Wyoming (17). The pre-industrial level of mercury concentration is about 4 ng L-1 then in 1815 there is a spike corresponds to the eruption of a volcano at Tambora in Indonesia, which caused world-wide crop failures. The increase for the years 1850-1875 corresponds to the gold rush and the rise after World War I to the growth in industrial use which declined sharply after about 1985.
While metallic mercury may not be particularly toxic by ingestion, being used in former times as a laxative, probably the only reusable laxative, since it is not readily absorbed, inhalation of the vapour has long been known to cause adverse effects. This was well illustrated during the construction of St Isaac’s Cathedral in Saint Petersburg where 100 kg of gold was used to gild a magnificent dome. By the time the construction was complete in 1858, at least sixty workers had died, and possible many more, from mercury poisoning: mercury was used as a solvent for gold (18). When the Soviets took over, the rich contents of the cathedral were confiscated and the building was converted into the Museum of Atheism, but it is now fully restored.
|
A selection of LD50 values for mercury compounds shows that Hg(I) compounds are usually less toxic than Hg(II), HgS is non-toxic by virtue of its insolubility and dimethyl mercury is by far the most toxic species.
Methyl mercury, CH3Hg+, was implicated in the notorious incidents at Minimata Bay in Japan. Nihon Chisso Hiryo–Kabushiki Kaisha (Japan Nitrogenous Fertilizer Company) was set up in 1908 to utilise hydroelectric power to make calcium carbide which was converted into calcium cyanamide and then to calcium nitrate. In addition, the calcium carbide was used as a source of acetylene which was used to manufacture acetaldehyde between 1932 and 1968 using mercury catalysis. Waste water containing CH3Hg+ or Hg2+ was discharged into the sea where the Hg2+ was biomethylated by microorganisms to give CH3Hg+ which was bioconcentrated, ending up in fish which was used for human consumption, leading to poisonings and deaths over a period of years (19,20). Cats were the first to die. They would dance on their hind legs through the streets before plunging into the sea. Cats are enthusiastic fish eaters. Methyl mercury readily crosses the blood-brain barrier, causes ataxia, sensory disturbance and changes in mental state, birth defects and inhibits several stages of neurotransmission in the brain. It is very slowly excreted from the body, unlike inorganic mercury, which has a half-life of about a month. The powerful toxicity of dimethyl mercury was discovered soon after it was first prepared (1852), causing several deaths in 1865 (21).
|
Potential exposure of the public in Europe to metallic mercury has led to the banning of mercury in glass thermometers (Directive 2007/51/EC), except for scientific purposes, and a recommendation that the emission of mercury from crematoria be cut by 50% by 2012. To achieve this aim, there appears to be two options: (i) fit expensive filters to remove the mercury, which has the on-going expense of collection and disposal of the metal and (ii) close half of the crematoria. There has been a suggestion, from Sweden, that mercury should be captured as HgS which has a very low toxicity due to its insolubility, followed by geological long-term storage.
Lead
Although Pliny noted the toxicity resulting from lead smelting (22)
“While it is being melted, all the apertures in the vessel should be closed, otherwise a noxious vapour is discharged from the furnace, of a deadly nature, to dogs in particular.”
and it was generally thought that the use of lead containers to concentrate grape juice created a toxicity hazard, but it is now suggested that chronic lead poisoning did not contribute significantly to the fall of the Roman Empire in the West (23,24).
But the use of lead containers for food or drink was a source of chronic toxicity for many years. In the 18th century, Devonshire colic was thought to be caused by using lead containers in brewing cider, although this was disputed. Some breweries in the early 19th century advertised their beer as being lead-free. But such a case was reported in the British Medical Journal by a doctor in Morriston, Glamorganshire, in 1900 (25). A man, just referred to as “W.O.”, a wheelwright and carpenter in a tin works, became ill:
“At this time [June 1898] there was great prostration and general muscular trembling; he could not stand, he could not lift a cup with his hand, and had scarcely sufficient control of the facial muscles to speak; there was constant vomiting, persistent constipation, pains all over, and progressive loss of flesh.”
The cause was unclear for some time. “I then for the first time examined the gums, when at once the explanation of everything became plain, for there was a very decided blue line” – a symptom of lead poisoning. After extensive investigation, the source was revealed. “W.O.” on his way to work and on his return, six times a day, was in the habit of taking refreshment at a public house and was always the first customer at 6 a.m. His first pint of the day was beer which had stood overnight in the 20 feet of lead pipe between the cellar and the tap. Analysis showed that this contained lead which proved fatal: “The last I heard of him was that in the following January he fell down in a fit and lingered a few hours before he died.”
Friedrich Christian Accum (1769-1838) drew attention to the use of lead in food, for example, lead chromate to enhance the colour of turmeric, in 1820 (26). But effective legislation was a long time coming. Lead in solder for food and drink tins was only banned in the second half of the 20th century.
In contrast to arsenic and mercury, alkylated lead compounds are not generated in the environment. Ethyl lead compounds were first synthesized by Carl Jacob Löwig (1803-1890) in 1853 by adding iodoethane to a 1:6 alloy of sodium and lead and gently heating (27). Tetraethyl lead remained a compound of only academic interest for the next 60 years. Thomas Midgley (1889-1944) was an engineer working at the Dayton Research Laboratories, a subsidiary of General Motors, and searching for an anti-knock agent for automobile engines. Years later, he described his success (28)
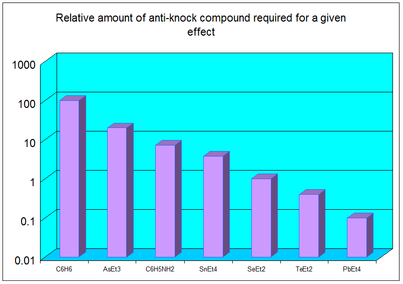
“[...] we profitably abandoned the Edisonian method in favor of a correlation procedure based on the periodic table. ... Predictions began fulfilling themselves instead of fizzling. Diethyl selenide was prepared and worked as expected; diethyl telluride next fulfilled our predictions. ... The results were plotted as anti-knock effect vs. atomic number. Tin was the first element investigated from the group immediately to the left of those previously reported. Its ethyl derivative was studied. This compound exhibited a much more powerful effect than had been expected. We thereupon predicted that tetraethyl lead would solve the problem.”
Although Pliny noted the toxicity resulting from lead smelting (22)
“While it is being melted, all the apertures in the vessel should be closed, otherwise a noxious vapour is discharged from the furnace, of a deadly nature, to dogs in particular.”
and it was generally thought that the use of lead containers to concentrate grape juice created a toxicity hazard, but it is now suggested that chronic lead poisoning did not contribute significantly to the fall of the Roman Empire in the West (23,24).
But the use of lead containers for food or drink was a source of chronic toxicity for many years. In the 18th century, Devonshire colic was thought to be caused by using lead containers in brewing cider, although this was disputed. Some breweries in the early 19th century advertised their beer as being lead-free. But such a case was reported in the British Medical Journal by a doctor in Morriston, Glamorganshire, in 1900 (25). A man, just referred to as “W.O.”, a wheelwright and carpenter in a tin works, became ill:
“At this time [June 1898] there was great prostration and general muscular trembling; he could not stand, he could not lift a cup with his hand, and had scarcely sufficient control of the facial muscles to speak; there was constant vomiting, persistent constipation, pains all over, and progressive loss of flesh.”
The cause was unclear for some time. “I then for the first time examined the gums, when at once the explanation of everything became plain, for there was a very decided blue line” – a symptom of lead poisoning. After extensive investigation, the source was revealed. “W.O.” on his way to work and on his return, six times a day, was in the habit of taking refreshment at a public house and was always the first customer at 6 a.m. His first pint of the day was beer which had stood overnight in the 20 feet of lead pipe between the cellar and the tap. Analysis showed that this contained lead which proved fatal: “The last I heard of him was that in the following January he fell down in a fit and lingered a few hours before he died.”
Friedrich Christian Accum (1769-1838) drew attention to the use of lead in food, for example, lead chromate to enhance the colour of turmeric, in 1820 (26). But effective legislation was a long time coming. Lead in solder for food and drink tins was only banned in the second half of the 20th century.
In contrast to arsenic and mercury, alkylated lead compounds are not generated in the environment. Ethyl lead compounds were first synthesized by Carl Jacob Löwig (1803-1890) in 1853 by adding iodoethane to a 1:6 alloy of sodium and lead and gently heating (27). Tetraethyl lead remained a compound of only academic interest for the next 60 years. Thomas Midgley (1889-1944) was an engineer working at the Dayton Research Laboratories, a subsidiary of General Motors, and searching for an anti-knock agent for automobile engines. Years later, he described his success (28)
“[...] we profitably abandoned the Edisonian method in favor of a correlation procedure based on the periodic table. ... Predictions began fulfilling themselves instead of fizzling. Diethyl selenide was prepared and worked as expected; diethyl telluride next fulfilled our predictions. ... The results were plotted as anti-knock effect vs. atomic number. Tin was the first element investigated from the group immediately to the left of those previously reported. Its ethyl derivative was studied. This compound exhibited a much more powerful effect than had been expected. We thereupon predicted that tetraethyl lead would solve the problem.”
|
The results he obtained are illustrated in Figure 1 (29).
Midgley promoted tetraethyllead (TEL) as a superior anti-knock agent to ethanol, and it was more profitable too. Production commenced in 1923 but was soon temporarily halted when production workers became ill (hallucinations, madness) and died. Midgley needed a holiday in Florida to recover from the effects after demonstrating the safety of TEL. Following improvements in the handling, and studies funded by the Ethyl Corporation, which proved that TEL was safe, production resumed. Posters announced ‘Ethyl is back’. It stayed until Congress finally outlawed it completely (in the US) in 1989. Later in life, Midgley introduced the world to chlorofluorocarbon refrigerant gases (30). |
|
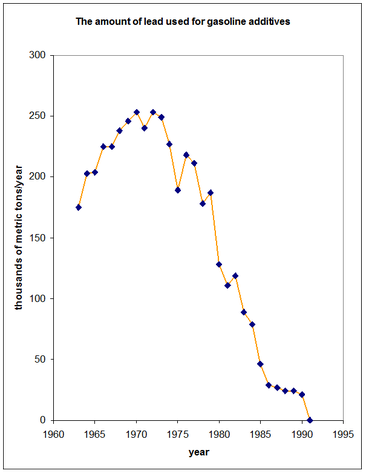
The year before TEL was unleashed on the world, Clair Cameron Patterson (1922-1995) was born, an Iowa farm boy, in spite of his first name. Researching at the University of Chicago, he elected to work on determining the uranium/lead isotopic ratios, using mass spectrometry, in old minerals with a view to estimating the age of the Earth. He concluded, in 1953, that the answer was 4550 million years, a figure that still stands today. His big problem was to find minerals which were not contaminated with modern lead and then to do measurements in an uncontaminated environment. The source of the contamination, he decided, was from lead in petrol and he campaigned for many years for its removal, attracting the wrath of the big oil companies (31). He was eventually successful (32). This is illustrated in Figure 2 by the amount of lead used in gasoline additives over a 30-year period (33).
Production of TEL ceased in the US and Canada and was moved to Ellesmere Port in Cheshire, where it continued for a few years until, following fires and explosions, it was moved elsewhere. Following the removal of lead in petrol in the US, a 90% reduction in the mean blood lead level concentration occurred between 1976 and 1995. |
Cadmium
In contrast to arsenic, mercury and lead, cadmium is a recent discovery (1817). The discoverers were either Karl Samuel Leberecht Hermann (1765-1846) or Friedrich Stromeyer (1776-1835). It is usually associated with zinc. Cadmium was widely used in plating to protect other metals from corrosion and as a pigment (CdS, CdSe). The toxicity of cadmium compounds was first reported in 1858 when it was found that exposure to cadmium carbonate dust used in silver polishing caused respiratory and gastrointestinal symptoms (34). The first chronic poisoning, causing rhinitis and pharyngitis in plating workers, was reported in 1940 (35). By the 1950s the hazards of working with cadmium were well established, causing emphysema and proteinuria from inhalation (36).
Chronic poisoning by cadmium has been reported at several locations worldwide (37). The symptoms are renal osteomalacia caused by a build-up of cadmium in the kidneys leading to a bone disease with fractures and severe pain (38). The most notorious case occurred in Japan where the disease is known as itai-itai (Ouch! Ouch!). In 1910, the Mitsui Mining & Smelting Co Ltd started operations at the Kamioka Mines in the Toyama Prefecture where lead, copper and zinc were the major products. Waste water was discharged into the Jinzu River. The first poisoning was reported in 1912. Between 1939 and 1954, 200 people were affected by itai-itai and 100 died, but the cause of the illness was unknown at that time. In a December 1957 medical symposium in the Toyama Prefecture, a participating doctor, Dr. Noboru Hagino, suggested in a paper that itai-itai disease was caused by waste water from the factories. By 1961, he had narrowed down the source to cadmium discharged during mining activities in Kamioka by the Mitsui Mining Company (39). The evidence was convincing. Comparing the incidence (prevalence) of itai-itai with the level of cadmium pollution showed a high correlation (40).
The World Health Organization in its International Program on Chemical Safety, WHO/IPCS, reports that a drinking-water guideline value of 0.005 mg/litre for cadmium was set in 1984 (41).
In contrast to arsenic, mercury and lead, cadmium is a recent discovery (1817). The discoverers were either Karl Samuel Leberecht Hermann (1765-1846) or Friedrich Stromeyer (1776-1835). It is usually associated with zinc. Cadmium was widely used in plating to protect other metals from corrosion and as a pigment (CdS, CdSe). The toxicity of cadmium compounds was first reported in 1858 when it was found that exposure to cadmium carbonate dust used in silver polishing caused respiratory and gastrointestinal symptoms (34). The first chronic poisoning, causing rhinitis and pharyngitis in plating workers, was reported in 1940 (35). By the 1950s the hazards of working with cadmium were well established, causing emphysema and proteinuria from inhalation (36).
Chronic poisoning by cadmium has been reported at several locations worldwide (37). The symptoms are renal osteomalacia caused by a build-up of cadmium in the kidneys leading to a bone disease with fractures and severe pain (38). The most notorious case occurred in Japan where the disease is known as itai-itai (Ouch! Ouch!). In 1910, the Mitsui Mining & Smelting Co Ltd started operations at the Kamioka Mines in the Toyama Prefecture where lead, copper and zinc were the major products. Waste water was discharged into the Jinzu River. The first poisoning was reported in 1912. Between 1939 and 1954, 200 people were affected by itai-itai and 100 died, but the cause of the illness was unknown at that time. In a December 1957 medical symposium in the Toyama Prefecture, a participating doctor, Dr. Noboru Hagino, suggested in a paper that itai-itai disease was caused by waste water from the factories. By 1961, he had narrowed down the source to cadmium discharged during mining activities in Kamioka by the Mitsui Mining Company (39). The evidence was convincing. Comparing the incidence (prevalence) of itai-itai with the level of cadmium pollution showed a high correlation (40).
The World Health Organization in its International Program on Chemical Safety, WHO/IPCS, reports that a drinking-water guideline value of 0.005 mg/litre for cadmium was set in 1984 (41).
Conclusion
The health concerns of heavy metals and metalloids have been evident for a long time, but were often disregarded when commercial gain took precedence.
References
1. Trevan, J. W. The error of determination of toxicity. Proceedings of the Royal Society of London, 1927, 101B, 483-514.
2. Hodge, H. C. and Sterner, J. H. Tabulation of toxicity classes. American Industrial Hygiene Association Quarterly, 1949, 10(4), 93-96.
3. CLP-Regulation (EC) No 1272/2008.
4. McCann, M. Arsenic and other preservatives in museum specimens. Art Hazards News, 1995, 18(2), 1-2.
5. Stellman, J. M., Stellman, S. D., Christian, R., Weber, T. and Tomasallo, C. The Extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature, 2003, 422, 681-687.
6. Bartrip, P. A. “Pennurth of Arsenic for Rat Poison”: The Arsenic Act, 1851 and the prevention of secret poisoning. Medical History, 1992, 36, 53-69.
7. Anon. The production of aniline colours without the use of arsenic acid. Chemical News, 1875, 31, 40.
8. Yamauchi, H., Kaise, T., Takahashi, K. and Yamamura, Y. Toxicity and metabolism of trimethylarsine in mice and hamsters. Fundamental Applied Toxicology, 1990, 14, 399-407.
9. Yamanaka, K., Hoshino, M., Okamoto, M., Sawamura, R., Hasegawa, A. and Okada S. Induction of DNA damage by dimethylarsine, a metabolite of inorganic arsenics, is for the major part likely due to its peroxyl radical. Biochemical and Biophysics Research Communications, 1990, 168(1), 58-64.
10. The author, personal observation.
11. Cullen, W. R. and Reimer, K. J. Arsenic speciation in the environment. Chemical Reviews, 1989, 89, 713-764.
12. Jones, D. E. H. and Ledingham, K. W. D. Arsenic in Napoleon’s wallpaper. Nature, 1982, 299, 626-627.
13. Cullen, W. R. and Bentley, R. The toxicity of trimethylarsine: an urban myth. Journal of Environmental Monitoring, 2005, 7, 11-15.
14. Hutchinson, J. Arsenic cancer. British Medical Journal, 1887, 2, 1280.
15. Neubauer, O. Arsenical cancer: A review. British Journal of Cancer. 1947, 1(2), 192-251.
16. Environmental Health Criteria 224 : ARSENIC AND ARSENIC COMPOUNDS, 2nd edn., pp 501, World Health Organization, Geneva, 2001.
17. Schuster, P. F., Krabbenhoft, D. P., Naftz, D. L., Cecil, L. D., Olson, M. L., Dewild, J. F., Susong, D. D., Green, J. R. and Abbott, M. L. Atmospheric mercury deposition during the last 270 Years: A glacial ice core record of natural and anthropogenic sources. Environmental Science and Technology, 2002, 36(11), 2303-2310.
18. http://www.nevsky-prospekt.com/isaacs.html (accessed 25 November 2011).
19. Thayer, J. S. Biological methylation – its nature and scope, Journal of Chemical Education, 1973, 50(6), 390-391.
20. Jensen, S. and Jernelöv, A. Biological methylation of mercury in aquatic organisms. Nature, 1969, 223, 753; doi:10.1038/223753a0.
21. Edwards, G. N. Two cases of poisoning by mercuric methide. St Bartholomew’s Hospital Reports, Vol. 1, Longmans, Green, and Co., London, 1865, pp 141-150.
22. Pliny the Elder. Historia Naturalis, xxxiv, 50, 167.
23. Retief, F. P. and Cilliers L. Lead poisoning in Ancient Rome. Acta Theologica, 2006, 26(2), 147-164.
24. Scarborough, J. The myth of lead poisoning among Romans: an essay reviewed, Journal of the History of Medicine, 1984, 39, 469-75.
25. Morgan, E. R. A case of lead poisoning by beer. British Medical Journal, 1900, 1373.
26. Accum, F. C. A treatise on adulterations of food and culinary poisons, exhibiting the fraudulent sophistications of bread, beer, wine, spirituous liquors, tea, coffee, cream, confectionary, vinegar, mustard, pepper, cheese, olive oil, pickles, and other articles employed in domestic economy, and methods of detecting them. Milner and Company, London, 1820.
27. Löwig, C. J. Ueber Methplumbäthyl. Annalen der Chemie, 1853, 88, 318-323; Journal für Praktische Chemie, 1853, 60, 304-310.
28. Nickerson, S. P. Tetraethyl Lead: A product of American research. Journal of Chemical Education, 1954, 31, 569.
29. Replotted on a logarithmic scale from data in reference 28.
30. “Leaded Gasoline, Safe Refrigeration, and Thomas Midgley, Jr.” Chapter 6 in McGrayne, S. B., Prometheans in the Lab: Chemistry and the Making of the Modern World, McGraw-Hill, New York, 2002; ISBN 0-07-140795-2.
31. Patterson, C. C. Contaminated and natural lead environments of man. Archives of Environmental Health, 1965, 11, 344-360.
32. Bryson, B. “Getting the lead out” in A Short History of Nearly Everything, Black Swan, London 2004, pp 193-205.
33. Data from http://minerals.usgs.gov/minerals/pubs/commodity/lead/stat/tbl2.txt; 1987-91 U.S. Department of Commerce estimate.
34. Sovet, A. Poisoning caused by powder used in cleaning of silver. Presse Med. Belge., 1858, 10, 69-70.
35. Mancioli, G. Le alterazioni rinofaringee nei lavoratori del cadmio. Rassegna di medicina industriale e di igiene del lavoro, 1940, 11, 632.
36. Bonnell, J. A. Emphysema and proteinuria in men casting copper-cadmium alloys. British Journal of Industrial Medicine, 1955, 12, 181.
37. Kjellstrom, T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environmental Health Perspectives, 1979, 28, 169-197.
38. Nordberg, G. F. New insights into the mechanisms of cadmium toxicity: Advances in cadmium research. Toxicology and Applied Pharmacology, 2009, 238(3), 192-200.
39. Makoto, K. Research history of Itai-Itai disease for 56 years and future problems in the 21st Century. Itaiitaibyo Shori Hanketsu 30shunen Shinpojiumu, 2003, 5-20. (Japanese; English abstract) http://sciencelinks.jp/j-east/article/200311/000020031103A0341096.php
40. http://www1.wlsh.tyc.edu.tw/~globalschoolnet/a6.html (accessed 4 December 2011)
41. Cadmium. Environmental Health Criteria Document 134, IPCS. WHO, Geneva, 1992, pp 1-280.
The health concerns of heavy metals and metalloids have been evident for a long time, but were often disregarded when commercial gain took precedence.
References
1. Trevan, J. W. The error of determination of toxicity. Proceedings of the Royal Society of London, 1927, 101B, 483-514.
2. Hodge, H. C. and Sterner, J. H. Tabulation of toxicity classes. American Industrial Hygiene Association Quarterly, 1949, 10(4), 93-96.
3. CLP-Regulation (EC) No 1272/2008.
4. McCann, M. Arsenic and other preservatives in museum specimens. Art Hazards News, 1995, 18(2), 1-2.
5. Stellman, J. M., Stellman, S. D., Christian, R., Weber, T. and Tomasallo, C. The Extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature, 2003, 422, 681-687.
6. Bartrip, P. A. “Pennurth of Arsenic for Rat Poison”: The Arsenic Act, 1851 and the prevention of secret poisoning. Medical History, 1992, 36, 53-69.
7. Anon. The production of aniline colours without the use of arsenic acid. Chemical News, 1875, 31, 40.
8. Yamauchi, H., Kaise, T., Takahashi, K. and Yamamura, Y. Toxicity and metabolism of trimethylarsine in mice and hamsters. Fundamental Applied Toxicology, 1990, 14, 399-407.
9. Yamanaka, K., Hoshino, M., Okamoto, M., Sawamura, R., Hasegawa, A. and Okada S. Induction of DNA damage by dimethylarsine, a metabolite of inorganic arsenics, is for the major part likely due to its peroxyl radical. Biochemical and Biophysics Research Communications, 1990, 168(1), 58-64.
10. The author, personal observation.
11. Cullen, W. R. and Reimer, K. J. Arsenic speciation in the environment. Chemical Reviews, 1989, 89, 713-764.
12. Jones, D. E. H. and Ledingham, K. W. D. Arsenic in Napoleon’s wallpaper. Nature, 1982, 299, 626-627.
13. Cullen, W. R. and Bentley, R. The toxicity of trimethylarsine: an urban myth. Journal of Environmental Monitoring, 2005, 7, 11-15.
14. Hutchinson, J. Arsenic cancer. British Medical Journal, 1887, 2, 1280.
15. Neubauer, O. Arsenical cancer: A review. British Journal of Cancer. 1947, 1(2), 192-251.
16. Environmental Health Criteria 224 : ARSENIC AND ARSENIC COMPOUNDS, 2nd edn., pp 501, World Health Organization, Geneva, 2001.
17. Schuster, P. F., Krabbenhoft, D. P., Naftz, D. L., Cecil, L. D., Olson, M. L., Dewild, J. F., Susong, D. D., Green, J. R. and Abbott, M. L. Atmospheric mercury deposition during the last 270 Years: A glacial ice core record of natural and anthropogenic sources. Environmental Science and Technology, 2002, 36(11), 2303-2310.
18. http://www.nevsky-prospekt.com/isaacs.html (accessed 25 November 2011).
19. Thayer, J. S. Biological methylation – its nature and scope, Journal of Chemical Education, 1973, 50(6), 390-391.
20. Jensen, S. and Jernelöv, A. Biological methylation of mercury in aquatic organisms. Nature, 1969, 223, 753; doi:10.1038/223753a0.
21. Edwards, G. N. Two cases of poisoning by mercuric methide. St Bartholomew’s Hospital Reports, Vol. 1, Longmans, Green, and Co., London, 1865, pp 141-150.
22. Pliny the Elder. Historia Naturalis, xxxiv, 50, 167.
23. Retief, F. P. and Cilliers L. Lead poisoning in Ancient Rome. Acta Theologica, 2006, 26(2), 147-164.
24. Scarborough, J. The myth of lead poisoning among Romans: an essay reviewed, Journal of the History of Medicine, 1984, 39, 469-75.
25. Morgan, E. R. A case of lead poisoning by beer. British Medical Journal, 1900, 1373.
26. Accum, F. C. A treatise on adulterations of food and culinary poisons, exhibiting the fraudulent sophistications of bread, beer, wine, spirituous liquors, tea, coffee, cream, confectionary, vinegar, mustard, pepper, cheese, olive oil, pickles, and other articles employed in domestic economy, and methods of detecting them. Milner and Company, London, 1820.
27. Löwig, C. J. Ueber Methplumbäthyl. Annalen der Chemie, 1853, 88, 318-323; Journal für Praktische Chemie, 1853, 60, 304-310.
28. Nickerson, S. P. Tetraethyl Lead: A product of American research. Journal of Chemical Education, 1954, 31, 569.
29. Replotted on a logarithmic scale from data in reference 28.
30. “Leaded Gasoline, Safe Refrigeration, and Thomas Midgley, Jr.” Chapter 6 in McGrayne, S. B., Prometheans in the Lab: Chemistry and the Making of the Modern World, McGraw-Hill, New York, 2002; ISBN 0-07-140795-2.
31. Patterson, C. C. Contaminated and natural lead environments of man. Archives of Environmental Health, 1965, 11, 344-360.
32. Bryson, B. “Getting the lead out” in A Short History of Nearly Everything, Black Swan, London 2004, pp 193-205.
33. Data from http://minerals.usgs.gov/minerals/pubs/commodity/lead/stat/tbl2.txt; 1987-91 U.S. Department of Commerce estimate.
34. Sovet, A. Poisoning caused by powder used in cleaning of silver. Presse Med. Belge., 1858, 10, 69-70.
35. Mancioli, G. Le alterazioni rinofaringee nei lavoratori del cadmio. Rassegna di medicina industriale e di igiene del lavoro, 1940, 11, 632.
36. Bonnell, J. A. Emphysema and proteinuria in men casting copper-cadmium alloys. British Journal of Industrial Medicine, 1955, 12, 181.
37. Kjellstrom, T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environmental Health Perspectives, 1979, 28, 169-197.
38. Nordberg, G. F. New insights into the mechanisms of cadmium toxicity: Advances in cadmium research. Toxicology and Applied Pharmacology, 2009, 238(3), 192-200.
39. Makoto, K. Research history of Itai-Itai disease for 56 years and future problems in the 21st Century. Itaiitaibyo Shori Hanketsu 30shunen Shinpojiumu, 2003, 5-20. (Japanese; English abstract) http://sciencelinks.jp/j-east/article/200311/000020031103A0341096.php
40. http://www1.wlsh.tyc.edu.tw/~globalschoolnet/a6.html (accessed 4 December 2011)
41. Cadmium. Environmental Health Criteria Document 134, IPCS. WHO, Geneva, 1992, pp 1-280.
This article is based on a presentation by Chris Cooksey at the joint ECG/Historical Group Symposium ‘Environmental Chemistry: A Historical Perspective’ held at Burlington House on 26th October 2011