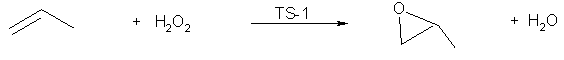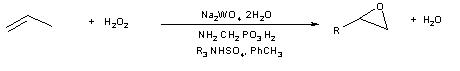Greener chemical manufacturing
James H. Clark
University of York
ECG bulletin January 2008
University of York
ECG bulletin January 2008
Professor James Clark, Director of the Green Chemistry Centre at the University of York, describes some of the new technologies that are emerging for chemical manufacturing as a result of the impact of increased raw materials and energy costs, new legislation, and environmental considerations.
Introduction
The chemical industry is under an unprecedented degree of pressure. Chemical manufacturers, suppliers and users face drivers to change at every stage in the supply chain:
· Market distortions in raw material price and availability due to the emerging mega-industries in the East
· Rapidly increasing oil prices affecting the feedstocks for over 90% of all organic chemicals and all chemical manufacturing
· Ever tighter legislation and punitive charges over hazardous chemical storage and waste disposal
· Increasing public and NGO pressure on chemical manufacturing and increasingly on chemical products. An exponential growth in chemical related legislation now especially affecting chemical substances (notably REACH).
While the 1990s saw a period of “good housekeeping” in the industry to improve the basic operating procedures, operator health and safety and relatively easy to deal with problems, there is a growing recognition that more changes are required including the introduction of new, cleaner technologies and careful examination of supply chains [1,2]. Examples of how industry, often in partnership with the research base, is addressing these challenges cover evaluation methods and technological solutions at all stages in the product supply chain. Some examples are described here.
Measuring green - Green Chemistry Metrics
The adage that if you can’t measure it, then you can’t fix it certainly applies to chemical production. One of the most important industry-led developments in greener chemical manufacturing in the last ten years has been the introduction of green chemistry process metrics [3]. It is now widely recognised that the measurement of yield alone is insufficient for reporting on process efficiency since it neglects process auxiliaries such as solvent, reagents and catalysts as well as the washing water, extraction solvent, drying agents, etc used to isolate the product.
One example of a green chemistry process metric is mass efficiency, which can be expressed as mass of final product divided by the total mass of all input materials. Very low mass efficiencies are currently found in higher value chemical manufacturing such as pharmaceuticals and speciality chemicals where multi-stage complex syntheses are used.
Green Chemistry Metrics can help to reveal major process inefficiencies such as high solvent usage or non-renewable reagents. Even these metrics may be inadequate since they tend not to include energy which in environmental and at today’s prices, economic terms is unreasonable. More sophisticated environmental footprinting will include energy consumption and can also cover extended manufacturing processes for example going beyond the primary manufacture of the active pharmaceutical ingredient to include the (secondary) manufacture of the fully-formulated products (e.g. tablets) and including packaging. In this way, chemical manufacturing companies will be able to better rectify hot spots of low resource efficiency, high waste or other specific problems.
Renewable resources
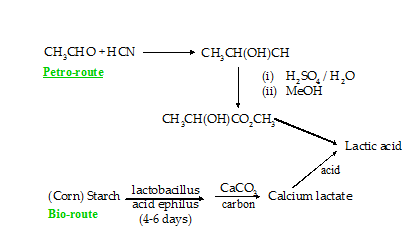
While over 90% of the organic chemical products on the market today are petroleum-derived, there is a rapidly growing interest in the use of alternative renewable resources, notably biomass. One of the best new commercial examples of this is bio-derived lactic acid, used now in large quantities for the manufacture of poly(lactic acid) [4]. ‘Bio-lactic’ acid is manufactured by NatureWorks L.L.C. (http://www.natureworksllc.com/) in a fermentation process starting with corn (Figure 1). One immediate concern with this is the competitive use of land and crops for non-food versus food production. In fact, lactic acid (as well as many other future biomass-derived building blocks chemicals) can also be made from waste biomass as has recently been demonstrated using sugarcane bagasse (India alone produces 45 million tonnes of this every year) [5]. In other respects the NatureWorks lactic acid manufacturing process appears to be a very good example of green chemistry: unlike the petrochemical based route, it is based on a renewable resource and from a wider environmental footprint perspective has few process hotspots.
Introduction
The chemical industry is under an unprecedented degree of pressure. Chemical manufacturers, suppliers and users face drivers to change at every stage in the supply chain:
· Market distortions in raw material price and availability due to the emerging mega-industries in the East
· Rapidly increasing oil prices affecting the feedstocks for over 90% of all organic chemicals and all chemical manufacturing
· Ever tighter legislation and punitive charges over hazardous chemical storage and waste disposal
· Increasing public and NGO pressure on chemical manufacturing and increasingly on chemical products. An exponential growth in chemical related legislation now especially affecting chemical substances (notably REACH).
While the 1990s saw a period of “good housekeeping” in the industry to improve the basic operating procedures, operator health and safety and relatively easy to deal with problems, there is a growing recognition that more changes are required including the introduction of new, cleaner technologies and careful examination of supply chains [1,2]. Examples of how industry, often in partnership with the research base, is addressing these challenges cover evaluation methods and technological solutions at all stages in the product supply chain. Some examples are described here.
Measuring green - Green Chemistry Metrics
The adage that if you can’t measure it, then you can’t fix it certainly applies to chemical production. One of the most important industry-led developments in greener chemical manufacturing in the last ten years has been the introduction of green chemistry process metrics [3]. It is now widely recognised that the measurement of yield alone is insufficient for reporting on process efficiency since it neglects process auxiliaries such as solvent, reagents and catalysts as well as the washing water, extraction solvent, drying agents, etc used to isolate the product.
One example of a green chemistry process metric is mass efficiency, which can be expressed as mass of final product divided by the total mass of all input materials. Very low mass efficiencies are currently found in higher value chemical manufacturing such as pharmaceuticals and speciality chemicals where multi-stage complex syntheses are used.
Green Chemistry Metrics can help to reveal major process inefficiencies such as high solvent usage or non-renewable reagents. Even these metrics may be inadequate since they tend not to include energy which in environmental and at today’s prices, economic terms is unreasonable. More sophisticated environmental footprinting will include energy consumption and can also cover extended manufacturing processes for example going beyond the primary manufacture of the active pharmaceutical ingredient to include the (secondary) manufacture of the fully-formulated products (e.g. tablets) and including packaging. In this way, chemical manufacturing companies will be able to better rectify hot spots of low resource efficiency, high waste or other specific problems.
Renewable resources
While over 90% of the organic chemical products on the market today are petroleum-derived, there is a rapidly growing interest in the use of alternative renewable resources, notably biomass. One of the best new commercial examples of this is bio-derived lactic acid, used now in large quantities for the manufacture of poly(lactic acid) [4]. ‘Bio-lactic’ acid is manufactured by NatureWorks L.L.C. (http://www.natureworksllc.com/) in a fermentation process starting with corn (Figure 1). One immediate concern with this is the competitive use of land and crops for non-food versus food production. In fact, lactic acid (as well as many other future biomass-derived building blocks chemicals) can also be made from waste biomass as has recently been demonstrated using sugarcane bagasse (India alone produces 45 million tonnes of this every year) [5]. In other respects the NatureWorks lactic acid manufacturing process appears to be a very good example of green chemistry: unlike the petrochemical based route, it is based on a renewable resource and from a wider environmental footprint perspective has few process hotspots.
|
Future biorefineries like the petroleum refineries of today will have energy as their main product(s). The sustainable chemicals of tomorrow can come from a relatively small fraction of the refinery turnover similar to petrochemicals today. Solvay has already demonstrated how a biofuel by-product can be used to make a chemical product that might be considered to be economically as well as environmentally beneficial [1]. The commodity chemical epichlorohydrin is made by a selective chlorodehydroxylation-dehydrochlorination process starting from the glycerine by-product from biodiesel manufacture, although the environmental benefits of at least some biodiesel feedstocks have been brought into question. It is important that green chemistry is flexible enough to take advantage of such opportunities, even if they don’t always turn out to be viable in the long term.
|
Cleaner Production
Green Chemistry Metrics can be used to identify the most wasteful chemical reactions. The simplest of these, Atom Economy, the sum of the molecular weights of the substrates divided by that of the desired product, shows large differences between a simple A + B = C reaction (e.g. Michael addition, hydrogenation) and those where only a small part of one or more of the substrates is incorporated in the desired product (e.g. oxidations using stoichiometric heavy metal oxidants and fluorinations using quaternary ammonium fluorides).
Green Chemistry Metrics can be used to identify the most wasteful chemical reactions. The simplest of these, Atom Economy, the sum of the molecular weights of the substrates divided by that of the desired product, shows large differences between a simple A + B = C reaction (e.g. Michael addition, hydrogenation) and those where only a small part of one or more of the substrates is incorporated in the desired product (e.g. oxidations using stoichiometric heavy metal oxidants and fluorinations using quaternary ammonium fluorides).
By using more elaborate metrics such as Mass Efficiency and E Factor, reactions that use large quantities of high molecular weight catalysts (e.g. AlCl3 in Friedel-Crafts acylations) and reactions that require protection/deprotection agents and activating groups (e.g. in amidations) are also exposed as especially wasteful.
Evaluations such as these helped a consortium of the world’s largest pharmaceutical manufacturers to prioritise common reaction types that need greening. The priorities included amide formation (without poor atom economy reagents such as acid activating groups) oxidations (without chlorinated solvents), Friedel-Crafts reactions (without stoichiometric “catalysts”), fluorination (with lower environmental impact non-hazardous processes) and reduction (without hydride reagents) [6].
One of these where progress at an industrial scale has been made is Friedel-Crafts acylation using a recoverable and safe-to-handle catalyst rather than a non-renewable and hazardous Lewis acid [7].
One of these where progress at an industrial scale has been made is Friedel-Crafts acylation using a recoverable and safe-to-handle catalyst rather than a non-renewable and hazardous Lewis acid [7].
|
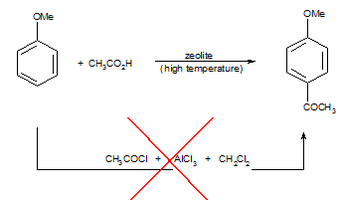
The zeolite route (Figure 2), developed by Rhodia shows dramatically improved green chemistry metrics (compared to the traditional AlCl3 route including a much lower E Factor and higher atom efficiency), as well as avoiding hot spots such as the use of a halogenated solvent and a dangerous reagent. However, the low activity of the zeolite catalyst limits effective reactions to those of activated substrates, and a more active “green” catalytic system is urgently needed.
Commercial progress has also been made with epoxidation reactions which represent one of the versatile ways of activating alkenes. With small molecules, the solid titanium silicate catalyst TS-1 developed by Enichem is very effective (Figure 3) [7]. The reaction has excellent green chemistry credentials with the use of a solid, renewable catalyst, a relatively benign and atom efficient oxidant and only water as the co-product: This can be considered to be a greener alternative to the commercially established but less atom efficient route based on organic hydroperoxides and transition metal catalysts [7]. |
Larger pore versions of the TS-1 catalyst show promise for future applications with larger molecules. Noyori’s chlorinated solvent-free and peracid-free epoxidation system (Figure 4) also lends itself to larger substrates and can be useful for higher value products such as fragrance and pharmaceutical intermediates [7].
New Green Technologies
The use of non-fossil feedstocks for chemical manufacture, alternative routes to important chemical products and the use of novel catalysts such as solid acids and porous solids represent step changes in reducing the environmental impact of chemical manufacturing. The associated reaction engineering is however, often conventional, e.g., batch type reactors or established continuous flow processing. A good example of novel, so-called “intensive processing” is the use of spinning disc reactors whereby the reaction fluids (e.g. a solution of substrates) are fed onto a rapidly spinning disc (which may have a catalytically active surface) [8] which can be heated or cooled. One of the key features is the short residence time in the reaction zone, reducing process hazards but limiting the technology to fast reactions. The most promising non-VOC solvent technology is supercritical fluids and especially supercritical carbon dioxide which is already proven on an industrial scale for extraction, e.g. decaffeination, and is now being reported in industrial chemical synthesis [9]. The main advantages of supercritical carbon dioxide in this context are non-toxicity and, in particular, ease of separation (the source of much of the waste in many chemical processes in the separation steps after reaction).
The other best-studied non-VOC-solvent technology is the use of non-volatile ionic liquids. Here the removal of the problem of atmospheric pollution is a prominent feature in the appeal of the use of these interesting substances, as is the flexibility of the liquid in terms of solvent power and activity (e.g. enabling the solvent to also act as a strong, typically acid catalyst). Here industrial development is progressing through industry-academic partnerships such as that involving Merck [10].
Another step-change technology that may be about to become widely used in chemical processing is microwave activation including microwave assisted organic reactions. While the technique has been a popular academic subject for a number of years, clear evidence for improved energy efficiency in chemical synthesis [11] has only recently been reported, and the emergence of a number of large scale microwave reactor manufacturing companies may open the ways to future large scale chemical manufacturing based on very short reaction times [12].
Conclusion
Unprecedented pressures for change across all stages in the lifecycle of chemical products provides a unique platform for the development and application of cleaner technologies that are more sustainable and reduce the overall environmental footprint of chemical products.
References
Professor JAMES H. CLARK,
Director of the Green Chemistry Centre,
University of York,
December 2007
www.greenchemistry.net
Biographical Note: James Clark has an international reputation for his work in green chemistry and was a founding director of the Green Chemistry Network at the University of York. He was also the founding Scientific Editor for the RSC journal Green Chemistry, and is an author of numerous books on green chemistry. He now holds the Chair of Industrial & Applied Chemistry and heads the Green Chemistry Centre at York, which integrates green chemistry research, industrial collaboration and educational developments and issues relevant to the public understanding of science. He is also the Director of the Green Chemistry Centre of Industrial Collaboration. His research interests include heterogeneous catalysis and supported reagents, and the exploitation of renewable resources.
The use of non-fossil feedstocks for chemical manufacture, alternative routes to important chemical products and the use of novel catalysts such as solid acids and porous solids represent step changes in reducing the environmental impact of chemical manufacturing. The associated reaction engineering is however, often conventional, e.g., batch type reactors or established continuous flow processing. A good example of novel, so-called “intensive processing” is the use of spinning disc reactors whereby the reaction fluids (e.g. a solution of substrates) are fed onto a rapidly spinning disc (which may have a catalytically active surface) [8] which can be heated or cooled. One of the key features is the short residence time in the reaction zone, reducing process hazards but limiting the technology to fast reactions. The most promising non-VOC solvent technology is supercritical fluids and especially supercritical carbon dioxide which is already proven on an industrial scale for extraction, e.g. decaffeination, and is now being reported in industrial chemical synthesis [9]. The main advantages of supercritical carbon dioxide in this context are non-toxicity and, in particular, ease of separation (the source of much of the waste in many chemical processes in the separation steps after reaction).
The other best-studied non-VOC-solvent technology is the use of non-volatile ionic liquids. Here the removal of the problem of atmospheric pollution is a prominent feature in the appeal of the use of these interesting substances, as is the flexibility of the liquid in terms of solvent power and activity (e.g. enabling the solvent to also act as a strong, typically acid catalyst). Here industrial development is progressing through industry-academic partnerships such as that involving Merck [10].
Another step-change technology that may be about to become widely used in chemical processing is microwave activation including microwave assisted organic reactions. While the technique has been a popular academic subject for a number of years, clear evidence for improved energy efficiency in chemical synthesis [11] has only recently been reported, and the emergence of a number of large scale microwave reactor manufacturing companies may open the ways to future large scale chemical manufacturing based on very short reaction times [12].
Conclusion
Unprecedented pressures for change across all stages in the lifecycle of chemical products provides a unique platform for the development and application of cleaner technologies that are more sustainable and reduce the overall environmental footprint of chemical products.
References
- J. H. Clark, Green chemistry for the second generation biorefinery - sustainable chemical manufacturing based on biomass, J. Chem. Technol. Biotechnol., 2007, 82, 603.
- J. H. Clark, Green chemistry: today (and tomorrow), Green Chem., 2006, 8, 17.
- P. J. C. Constable et al, Green chemistry measures for process research and development, Green Chem., 2001, 3, 7.
- J. H. Clark and J. E. Hardy, Towards Sustainable Chemical Manufacturing: Polylactic Acid - A Sustainable Polymer? In Sustainable Development in Practice, A. Azapagic, S. Perdan and R. Clift (eds.), J. Wiley, Chichester, 2004, p 250.
- M. G. Adsul, A. J. Varma and D. V. Gokhale, Lactic acid production from waste sugarcane bagasse derived cellulose, Green Chem., 2007, 9, 58.
- D. J. C. Constable et al, Key green chemistry research areas - a perspective from pharmaceutical manufacturers, Green Chem., 2007, 9, 411.
- Green Chemistry and Catalysis, R. A. Sheldon, I. Arends and U. Hanefield (eds.), Wiley-VCH, Weinheim, Germany, 2007.
- See for example: K. V. K. Boodhoo, W. A. E. Dunk, M. Vicevic et al, Classical cationic polymerization of styrene in a spinning disc reactor using silica-supported BF3 catalyst, J. Appl. Polym. Sci., 2006, 101, 8.
- P. Licence, J. Ke, M. Sokolova, S. K. Ross and M. Poliakoff, Chemical reactions in supercritical carbon dioxide: from laboratory to commercial plant, Green Chem., 2003, 2, 99.
- S. Stolte et al, Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids, Green Chem., 2007, 9, 1170.
- M. J. Gronnow, R. J. White, J. H. Clark and D. J. Macquarrie, Energy Efficiency in Chemical Reactions: A Comparative Study of Different Reaction Techniques, Org. Process Res. Dev., 2005, 9, 516.
- See for example: Industrial Microwave Systems L.L.C. web site http://www.industrialmicrowave.com
Professor JAMES H. CLARK,
Director of the Green Chemistry Centre,
University of York,
December 2007
www.greenchemistry.net
Biographical Note: James Clark has an international reputation for his work in green chemistry and was a founding director of the Green Chemistry Network at the University of York. He was also the founding Scientific Editor for the RSC journal Green Chemistry, and is an author of numerous books on green chemistry. He now holds the Chair of Industrial & Applied Chemistry and heads the Green Chemistry Centre at York, which integrates green chemistry research, industrial collaboration and educational developments and issues relevant to the public understanding of science. He is also the Director of the Green Chemistry Centre of Industrial Collaboration. His research interests include heterogeneous catalysis and supported reagents, and the exploitation of renewable resources.