FASTA and environmental monitoring in Kenya
Steven Lancaster and colleagues*
Foundation for Analytical Science & Technology in Africa
ECG Bulletin September 2011
Foundation for Analytical Science & Technology in Africa
ECG Bulletin September 2011
The Foundation for Analytical Science & Technology in Africa (FASTA) is a registered charity comprised of industrial and academic scientists from the UK’s analytical sector. It was founded by Steve Lancaster (Royal Society of Chemistry) and Barrie Nixon (Mass Spec UK) on September 20th, 2006 to support the development of promising scientists, analytical research and the preservation of the environment in Africa, via capacity-building and technology transfer. This was in response to a request for assistance from Professor Anthony Gachanja of Jomo Kenyatta University of Agriculture & Technology. Having observed a lack of analytical equipment and instrumentation, he noted that this posed an impediment to scientific progress and research in Africa. Several organisations, including: Mass Spec UK, The British Mass Spectrometry Society, The Analytical Chemistry Trust Fund, The Royal Society of Chemistry, Pan Africa Chemistry Network (PACN), Pfizer, BP and Perkin-Elmer, amongst others, have been very supportive by providing generous grants and donating equipment. Their support has been critical in allowing FASTA to set up our first partner GC-MS laboratory at Jomo Kenyatta University of Agriculture & Technology (JKUAT) in Nairobi, where Anthony is based. The PACN has also provided funding towards training courses and the purchase of instrumentation in both Nairobi and Addis Ababa, which in turn has resulted in the development of analytical centres of excellence at both locations.
The aims and objectives of FASTA are synergistic with the PACN initiative (to progress chemistry in Africa, recognising that analytical science is key in all areas of the chemical sciences) and are summarised below:
The aims and objectives of FASTA are synergistic with the PACN initiative (to progress chemistry in Africa, recognising that analytical science is key in all areas of the chemical sciences) and are summarised below:
- To facilitate research and teaching into chemical systems/processes and environmental processes;
- To enable/facilitate the provision of accurate, affordable and accessible environmental monitoring services at the local and national level;
- To promote and encourage food analysis in order to facilitate the import and export of foodstuffs that fully conform to human health and safety standards.
|
In this regard, FASTA is collaborating with Professor Gachanja and continually widening the scope of the laboratory. Since its inception, FASTA has invested a significant proportion of its resources into JKUAT, with the view that working closely and concertedly with a small number of facilities will ensure their success and, ultimately, that of other partner facilities. Indeed, the goal is for JKUAT, under the guidance of Professor Gachanja and the directors of FASTA, to become a hub for analytical science in East Africa, through technical excellence and high-level analytical equipment which can be used for analysis and training throughout the region (1-3).
Two GC-MS instruments are currently being used for postgraduate research and analysis of environmental samples at JKUAT. FASTA has also introduced thermal desorption equipment to be interfaced with one of the GC-MS. This will facilitate detailed atmospheric research and monitoring for a range of atmospheric pollutants. |
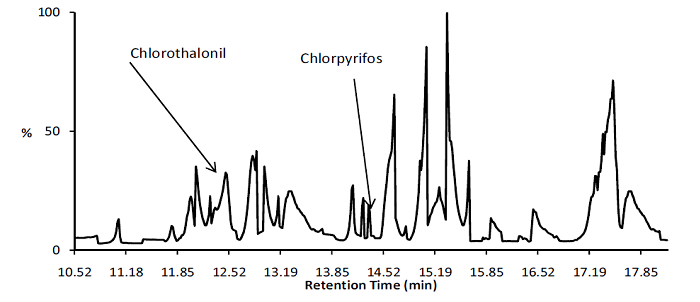
Some of the ongoing projects are: assessment of adulteration of fuels in Kenya; evaluation of quality of pharmaceuticals in the Kenyan market; monitoring and degradation of pesticides and other organic pollutants in the environment; and analysis of VOCs (volatile organic compounds) in local river water, using GC-MS. Figure 1 shows a GC-MS chromatogram of river water extract and identification of two pesticides used in coffee farms, following sample preparation and preconcentration by solid phase extraction. Through this work, JKUAT is now recognised by the Kenyan government as a centre of excellence in environmental research and analytical chemistry, and is engaged in a number of important analyses, e.g. determining presence and level of methanol in illicit (and dangerous) alcoholic beverages to resolve legal disputes. The sample throughput of a range of sample types is being facilitated by the use of a high throughput sample condenser to enable the concentration of multiple samples after extraction with organic solvents. These include pesticide residue samples in vegetables and honey, natural products from plants and the proposed concentration of forensic samples from wildlife.
FASTA and biodiversity conservation initiatives
Addressing the current environmental contamination issues that threaten wildlife and ecosystems in Africa will necessitate bridging the theoretical gap that often exists between analysts and conservationists and encouraging these stakeholders to merge their expertise. To achieve this, FASTA and Ngaio Richards, a forensic ecologist and wildlife conservationist, are working within a network of chemists and biologists. Together, they seek to develop the sampling and analytical chemistry structure required to gather sufficient data regarding the severe decline in Kenyan scavenger populations (e.g. vultures, lions and hyenas). Ngaio Richards’ doctoral research focused on the detection of nonsteroidal anti-inflammatory drugs (often abbreviated as NSAIDs) such as diclofenac (better known to arthritis sufferers as Voltarol/Voltaren) in alternative samples such as the hair of livestock animals and vulture feathers. (See Footnote 2) This research was inspired by a revelation which stunned the conservation community early in 2005, namely that vultures (Gyps species) on the Indian subcontinent were being poisoned by residues of diclofenac present in livestock carcasses (4,5). The drug was administered to the livestock to treat joint ailments/lameness, then carcasses were left out for the vultures to consume, and exposure induced visceral gout. So many vultures died that three species of Gyps were virtually extinguished.
Addressing the current environmental contamination issues that threaten wildlife and ecosystems in Africa will necessitate bridging the theoretical gap that often exists between analysts and conservationists and encouraging these stakeholders to merge their expertise. To achieve this, FASTA and Ngaio Richards, a forensic ecologist and wildlife conservationist, are working within a network of chemists and biologists. Together, they seek to develop the sampling and analytical chemistry structure required to gather sufficient data regarding the severe decline in Kenyan scavenger populations (e.g. vultures, lions and hyenas). Ngaio Richards’ doctoral research focused on the detection of nonsteroidal anti-inflammatory drugs (often abbreviated as NSAIDs) such as diclofenac (better known to arthritis sufferers as Voltarol/Voltaren) in alternative samples such as the hair of livestock animals and vulture feathers. (See Footnote 2) This research was inspired by a revelation which stunned the conservation community early in 2005, namely that vultures (Gyps species) on the Indian subcontinent were being poisoned by residues of diclofenac present in livestock carcasses (4,5). The drug was administered to the livestock to treat joint ailments/lameness, then carcasses were left out for the vultures to consume, and exposure induced visceral gout. So many vultures died that three species of Gyps were virtually extinguished.
Ngaio Richards approached FASTA in 2006 to discuss whether this research would be relevant to the analytical efforts underway to protect Kenya’s ailing vulture population, having learned about work being undertaken by FASTA from a previous report (3). However, after she had been in contact with a number of Kenya-based biologists and conservationists, it emerged that the most pressing threat to scavenging species was in fact pesticides used deliberately as poisons. A number of pesticides have been used for this purpose with the carbamate insecticide Carbofuran being particularly effective. This has been used at an alarming rate against a number of wildlife species (6,7). The habitat available for wild animals is increasingly limited and most of the remaining wildlife/nature reserves are immediately adjacent to populated and/or agricultural areas. Lions may prey on livestock (which appear to them a ready and easy source of food), and, in retaliation, ranchers or pastoralists may bait a carcass with Carbofuran to poison the lion, to prevent further decimation of livestock. Vultures, hyenas and jackals, among other scavengers, also gather at carcasses and are thus unintentionally poisoned as well. Numerous incidents of mass mortality have occurred, including one in which over one hundred vultures were poisoned. Many ‘nuisance’ animals such as baboons are also targeted (e.g. poison is injected into bananas) because they damage crops. There is also a growing concern for human health since, in some areas, Carbofuran is used for the practice of ‘pesticide hunting’ and ‘pesticide fishing’, whereby pesticides are used to bait and capture birds and mammals or sprinkled in water, for the purposes of collecting fish, for human consumption.
Unfortunately, there is not yet an organized monitoring system in place in Kenya, nor a specific protocol tailored to preserve the forensic integrity of samples collected in the field. Advanced analytical techniques which are able to provide conclusive and rapid forensic evidence are only available on a limited basis in many African countries. In Kenya, analysis of wildlife samples (which is not routinely carried out) tends to be by thin layer chromatography (TLC) only. While this technique is sensitive and appropriate as a first step, specific confirmation by HPLC or GC-MS is then required as a follow-up. Results should also be verified/confirmed at a second facility and there should ideally be an independent laboratory that could conduct quality assurance. Ultimately, the current situation has exposed the dearth of baseline data that exist on human and wildlife poisonings and shown the need to establish systematic and long-term monitoring of human and wildlife health in Kenya (and throughout Africa). FASTA hopes to address the need for more systematic environmental monitoring in a collaborative and concerted manner, by providing analytical support to and liaison between those engaged in such work.
Opportunities for collaboration in biodiversity conservation, environmental monitoring and capacity-building
The efforts to merge the analytical and conservation stakeholders attracted an Outreach Scholarship from the Analytical Chemistry Trust Fund, which enabled Ngaio Richards to spend 6 weeks in Kenya in the spring of 2010. The aim of this working visit was to meet face-to-face with Professor Gachanja and other key analytical researchers to explore the laboratory logistics and develop collaborations. Ngaio also spent several weeks in the field with various conservationists and researchers getting a sense of the challenges faced on-the-ground. The result was the development of a forensic field sampling protocol which is currently being reviewed by stakeholders. The protocol comprises a procedure for sampling several promising alternative matrices from carcasses (in addition to those that would otherwise be preferentially sampled if carcasses were found in good condition) and preserving samples (i.e. crop contents, stomach contents) when acute poisoning is suspected. An insect lifecycle timeline has also been developed (described below) to help estimate time since death and provide additional information to help determine whether or not poisoning was involved. (See Footnote).
If the monitoring of samples for environmental contaminants (e.g. Carbofuran) is to succeed on a long-term basis in Africa, it must essentially be self-sustaining. Although NSAIDs are not thought to represent a major threat to vultures and other scavengers in Kenya at this time, the background research on these drugs has nonetheless proven useful and will be used both advantageously and creatively. Given that NSAIDs can be administered to mask the signs of pain and inflammation, their use is closely monitored or in some cases prohibited in the horseracing industry. A collaboration is therefore under development between JKUAT and the Jockey Club of Kenya, whereby the laboratory would receive equine samples routinely collected on racing days to monitor for evidence of equine doping. Professor Gachanja is presently conducting a pilot project to integrate the NSAID detection methodology into a multi-screening process to monitor for evidence of equine doping. Protocol validation is already underway, and a student researcher is actively being sought to move the work forward. This collaboration would enable the JKUAT laboratory to generate a modest amount of revenue, which would in turn allow it to meet its operational costs and offer low cost analysis of wildlife samples to conservation bodies and other stakeholders with minimal financial resources. At the same time, there is a movement underway to comprehensively screen human and animal samples for evidence of exposure to a number of compounds of concern. This year, Ngaio and Anthony will also join forces with several Kenya-based research and conservation bodies to seek seed funding to pay for start-up costs.
A snapshot of recent developments in biodiversity conservation efforts in Kenya
In this section, we briefly outline a number of exciting and important initiatives that are currently underway in Kenya to conserve biodiversity and protect the environment. The directors of FASTA are convinced that integrating an analytical approach will greatly strengthen such initiatives and we will be pleased to offer our support as needed. This also provides us with an opportunity to showcase the outstanding efforts of our colleagues, who have managed to accomplish a great deal, often with relatively scant resources at their disposal.
Environmental monitoring and biodiversity conservation initiatives
For his MSc research, Peter Otieno, a secondary level chemistry teacher and a student at the University of Maseno, collected soil and water samples from two agricultural districts for analysis of Carbofuran residues and its primary metabolites. He also analysed the feet and beaks of several desiccated vultures that were recovered from the field. Residues of Carbofuran and/or primary metabolites were detected in all three types of samples (10,11). Peter’s work was conducted under the direction of Professor Joseph Lalah (at Nairobi Polytechnical University) and Dr Munir Z. Virani of the US-based The Peregrine Fund and the National Museums of Kenya. Peter’s current PhD research focuses on evaluating the performance of enzyme linked immunosorbent assay (ELISA) kits to assess the influence of climate change on the distribution of selected pesticide residues in sediment and water in Lake Naivasha. He will also evaluate the level of bioaccumulation of pesticide residues in fish species that are exposed to (elevated) residues levels in the aquatic ecosystem. ELISA kits are viewed by many as a viable analytical tool in monitoring and screening programs. The aim of the study is to generate accurate data that will be used to verify whether or not this is in fact the case for monitoring within the Kenyan (tropical) ecosystem.
Professor Joseph Lalah, who has recently been appointed as the Director of Research and Postgraduate Programmes for Nairobi Polytechnical University, is also involved in the development of integrated analytical approaches to assess indicators of the effectiveness of pesticide management practices at the catchment scale. For example, he is presently involved in an assessment of sugar cane farms in Nzoia area of Kenya where pesticides (particularly herbicides) are used intensively. This work is supported by the International Atomic Energy Agency through their coordinated research projects (CRPs). Professor Lalah is also interested in promoting the effective use of selected, non-persistent pesticides in mosquito larval control and is working on this with a student. In addition, he is supervising the work of a number of postgraduate students in the area of organic contaminants and inorganic contaminants (e.g. mercury), using HPLC/GC/GC-MS. Professor Lalah wishes to encourage efforts aimed at providing capacity for analytical techniques especially with respect to wildlife protection, environmental contamination and monitoring in Kenya and is very open to the kinds of partnerships and collaborative ventures that FASTA is keen to foster.
The Living with Lions (LWL: www.lionconservation.org/) programme, comprised of seven researchers and 34 Masaai warriors (the Lion Guardians, see: www.lionconservation.org/lion-guardians.html) are working to find ways for lions and local people to coexist and to ensure that the benefits of lions are made more tangible to local communities. There is now a growing concern that if the current rate of poisoning continues unabated, the lion population in Kenya faces the very real possibility of going extinct within the next five years. LWL has made great strides towards increasing the tolerance of communities towards lions, however finding ways to halt the use of poisons and their repercussions to the lion population is proving very difficult. The analytical infrastructure to detect and identify compounds that pose toxicological threats to lions (and vultures, discussed below) does not yet exist within Kenya, however this is an area that FASTA and JKUAT are actively working towards with the other partners in the network.
Kenyan vultures are on the decline and face a number of threats to their populations, which in turn has severe repercussions to ecosystem integrity (12). Dr Darcy Ogada has recently highlighted the important role played by vultures in fully decomposing carcasses (13). Using a series of observational and experimental trials with livestock carcasses, Dr Ogada and her colleagues found that in the absence of vultures mean carcass decomposition time more than tripled. Thus, vulture extinction could have far reaching ecological consequences and as they continue to decline due to poisoning and other human activities, increasing carcass consumption by mammalian scavengers may lead to carcasses becoming hubs of disease transmission within the scavenging carnivore community. In order to further understand the impacts of poisoning on vulture populations in Kenya, The Peregrine Fund and the Raptor Working Group of Nature Kenya, together with local wildlife conservancies, are planning a project in northern Kenya that will examine how land-use practices and human activities impact vulture populations. As part of this project, local attitudes and knowledge about vultures will be evaluated, to determine the extent livestock farmers understand the impacts of poisoning on wildlife, domestic animals and the environment. In October of this year, International Vulture Awareness Day celebrations will be held in Laikipia. Three hundred schoolchildren will be invited to perform dances, plays and songs about the importance of vultures. There will also be an art competition and lectures on the importance of conserving vultures and not using poisons. Participants will also be taken on a safari inside the conservancy to catch a glimpse of vultures in their natural habitats.
Dino Martins, an entomologist from Kenya who was recently awarded a PhD from Harvard, has been studying the repercussions of pesticides to beneficial insects, primarily honeybees/pollinators and dragonflies. Dino recently developed an insect lifecycle timeline (as far as we know, the first of its kind in Kenya) (14) to assist with establishing time of death at wildlife carcasses. Since insects are usually the first of the scavengers and decomposers to arrive at a carcass, proper observations of insects and their behaviour (particularly abnormal behaviour), mortality in and around a carcass and toxicological analysis of insects recovered from the carcass and immediate vicinity can help identify or narrow the compound(s) used. Insects can also be collected for toxicological analysis, to aid in positive identification of compounds and confirm suspicion of poisoning. Dino has also examined the effects of ‘pesticide fishing’ on dragonfly populations in Lake Victoria (15). His colleague Martin Odino, a conservation biologist affiliated with the National Museums of Kenya, has been conducting an extensive analysis of the repercussions of ‘pesticide hunting’ to migrant and resident birds in rice settlement schemes (7).
Unfortunately, there is not yet an organized monitoring system in place in Kenya, nor a specific protocol tailored to preserve the forensic integrity of samples collected in the field. Advanced analytical techniques which are able to provide conclusive and rapid forensic evidence are only available on a limited basis in many African countries. In Kenya, analysis of wildlife samples (which is not routinely carried out) tends to be by thin layer chromatography (TLC) only. While this technique is sensitive and appropriate as a first step, specific confirmation by HPLC or GC-MS is then required as a follow-up. Results should also be verified/confirmed at a second facility and there should ideally be an independent laboratory that could conduct quality assurance. Ultimately, the current situation has exposed the dearth of baseline data that exist on human and wildlife poisonings and shown the need to establish systematic and long-term monitoring of human and wildlife health in Kenya (and throughout Africa). FASTA hopes to address the need for more systematic environmental monitoring in a collaborative and concerted manner, by providing analytical support to and liaison between those engaged in such work.
Opportunities for collaboration in biodiversity conservation, environmental monitoring and capacity-building
The efforts to merge the analytical and conservation stakeholders attracted an Outreach Scholarship from the Analytical Chemistry Trust Fund, which enabled Ngaio Richards to spend 6 weeks in Kenya in the spring of 2010. The aim of this working visit was to meet face-to-face with Professor Gachanja and other key analytical researchers to explore the laboratory logistics and develop collaborations. Ngaio also spent several weeks in the field with various conservationists and researchers getting a sense of the challenges faced on-the-ground. The result was the development of a forensic field sampling protocol which is currently being reviewed by stakeholders. The protocol comprises a procedure for sampling several promising alternative matrices from carcasses (in addition to those that would otherwise be preferentially sampled if carcasses were found in good condition) and preserving samples (i.e. crop contents, stomach contents) when acute poisoning is suspected. An insect lifecycle timeline has also been developed (described below) to help estimate time since death and provide additional information to help determine whether or not poisoning was involved. (See Footnote).
If the monitoring of samples for environmental contaminants (e.g. Carbofuran) is to succeed on a long-term basis in Africa, it must essentially be self-sustaining. Although NSAIDs are not thought to represent a major threat to vultures and other scavengers in Kenya at this time, the background research on these drugs has nonetheless proven useful and will be used both advantageously and creatively. Given that NSAIDs can be administered to mask the signs of pain and inflammation, their use is closely monitored or in some cases prohibited in the horseracing industry. A collaboration is therefore under development between JKUAT and the Jockey Club of Kenya, whereby the laboratory would receive equine samples routinely collected on racing days to monitor for evidence of equine doping. Professor Gachanja is presently conducting a pilot project to integrate the NSAID detection methodology into a multi-screening process to monitor for evidence of equine doping. Protocol validation is already underway, and a student researcher is actively being sought to move the work forward. This collaboration would enable the JKUAT laboratory to generate a modest amount of revenue, which would in turn allow it to meet its operational costs and offer low cost analysis of wildlife samples to conservation bodies and other stakeholders with minimal financial resources. At the same time, there is a movement underway to comprehensively screen human and animal samples for evidence of exposure to a number of compounds of concern. This year, Ngaio and Anthony will also join forces with several Kenya-based research and conservation bodies to seek seed funding to pay for start-up costs.
A snapshot of recent developments in biodiversity conservation efforts in Kenya
In this section, we briefly outline a number of exciting and important initiatives that are currently underway in Kenya to conserve biodiversity and protect the environment. The directors of FASTA are convinced that integrating an analytical approach will greatly strengthen such initiatives and we will be pleased to offer our support as needed. This also provides us with an opportunity to showcase the outstanding efforts of our colleagues, who have managed to accomplish a great deal, often with relatively scant resources at their disposal.
Environmental monitoring and biodiversity conservation initiatives
For his MSc research, Peter Otieno, a secondary level chemistry teacher and a student at the University of Maseno, collected soil and water samples from two agricultural districts for analysis of Carbofuran residues and its primary metabolites. He also analysed the feet and beaks of several desiccated vultures that were recovered from the field. Residues of Carbofuran and/or primary metabolites were detected in all three types of samples (10,11). Peter’s work was conducted under the direction of Professor Joseph Lalah (at Nairobi Polytechnical University) and Dr Munir Z. Virani of the US-based The Peregrine Fund and the National Museums of Kenya. Peter’s current PhD research focuses on evaluating the performance of enzyme linked immunosorbent assay (ELISA) kits to assess the influence of climate change on the distribution of selected pesticide residues in sediment and water in Lake Naivasha. He will also evaluate the level of bioaccumulation of pesticide residues in fish species that are exposed to (elevated) residues levels in the aquatic ecosystem. ELISA kits are viewed by many as a viable analytical tool in monitoring and screening programs. The aim of the study is to generate accurate data that will be used to verify whether or not this is in fact the case for monitoring within the Kenyan (tropical) ecosystem.
Professor Joseph Lalah, who has recently been appointed as the Director of Research and Postgraduate Programmes for Nairobi Polytechnical University, is also involved in the development of integrated analytical approaches to assess indicators of the effectiveness of pesticide management practices at the catchment scale. For example, he is presently involved in an assessment of sugar cane farms in Nzoia area of Kenya where pesticides (particularly herbicides) are used intensively. This work is supported by the International Atomic Energy Agency through their coordinated research projects (CRPs). Professor Lalah is also interested in promoting the effective use of selected, non-persistent pesticides in mosquito larval control and is working on this with a student. In addition, he is supervising the work of a number of postgraduate students in the area of organic contaminants and inorganic contaminants (e.g. mercury), using HPLC/GC/GC-MS. Professor Lalah wishes to encourage efforts aimed at providing capacity for analytical techniques especially with respect to wildlife protection, environmental contamination and monitoring in Kenya and is very open to the kinds of partnerships and collaborative ventures that FASTA is keen to foster.
The Living with Lions (LWL: www.lionconservation.org/) programme, comprised of seven researchers and 34 Masaai warriors (the Lion Guardians, see: www.lionconservation.org/lion-guardians.html) are working to find ways for lions and local people to coexist and to ensure that the benefits of lions are made more tangible to local communities. There is now a growing concern that if the current rate of poisoning continues unabated, the lion population in Kenya faces the very real possibility of going extinct within the next five years. LWL has made great strides towards increasing the tolerance of communities towards lions, however finding ways to halt the use of poisons and their repercussions to the lion population is proving very difficult. The analytical infrastructure to detect and identify compounds that pose toxicological threats to lions (and vultures, discussed below) does not yet exist within Kenya, however this is an area that FASTA and JKUAT are actively working towards with the other partners in the network.
Kenyan vultures are on the decline and face a number of threats to their populations, which in turn has severe repercussions to ecosystem integrity (12). Dr Darcy Ogada has recently highlighted the important role played by vultures in fully decomposing carcasses (13). Using a series of observational and experimental trials with livestock carcasses, Dr Ogada and her colleagues found that in the absence of vultures mean carcass decomposition time more than tripled. Thus, vulture extinction could have far reaching ecological consequences and as they continue to decline due to poisoning and other human activities, increasing carcass consumption by mammalian scavengers may lead to carcasses becoming hubs of disease transmission within the scavenging carnivore community. In order to further understand the impacts of poisoning on vulture populations in Kenya, The Peregrine Fund and the Raptor Working Group of Nature Kenya, together with local wildlife conservancies, are planning a project in northern Kenya that will examine how land-use practices and human activities impact vulture populations. As part of this project, local attitudes and knowledge about vultures will be evaluated, to determine the extent livestock farmers understand the impacts of poisoning on wildlife, domestic animals and the environment. In October of this year, International Vulture Awareness Day celebrations will be held in Laikipia. Three hundred schoolchildren will be invited to perform dances, plays and songs about the importance of vultures. There will also be an art competition and lectures on the importance of conserving vultures and not using poisons. Participants will also be taken on a safari inside the conservancy to catch a glimpse of vultures in their natural habitats.
Dino Martins, an entomologist from Kenya who was recently awarded a PhD from Harvard, has been studying the repercussions of pesticides to beneficial insects, primarily honeybees/pollinators and dragonflies. Dino recently developed an insect lifecycle timeline (as far as we know, the first of its kind in Kenya) (14) to assist with establishing time of death at wildlife carcasses. Since insects are usually the first of the scavengers and decomposers to arrive at a carcass, proper observations of insects and their behaviour (particularly abnormal behaviour), mortality in and around a carcass and toxicological analysis of insects recovered from the carcass and immediate vicinity can help identify or narrow the compound(s) used. Insects can also be collected for toxicological analysis, to aid in positive identification of compounds and confirm suspicion of poisoning. Dino has also examined the effects of ‘pesticide fishing’ on dragonfly populations in Lake Victoria (15). His colleague Martin Odino, a conservation biologist affiliated with the National Museums of Kenya, has been conducting an extensive analysis of the repercussions of ‘pesticide hunting’ to migrant and resident birds in rice settlement schemes (7).
|
In addition, Dino (who has, incidentally, just been named an ‘Emerging Explorer’ by National Geographic: http://www.nationalgeographic.com/field/explorers/dino-martins/) is looking at the direct effects of pesticides on bees in Kenya. This is currently being drafted as a review of ‘routes to wild bee pesticide exposure’ for the bee families Halictidae and the Carpenter bees (Apidae). Preliminary data indicate that wild bee species are exposed to pesticides and suffer many negative effects from them. Many different aspects of wild bee biology are relevant to pesticide exposure including: nesting sites in soil that receive drainage water from crop fields, social behaviour where bees share nest sites/food resources and seasonality where bees forage intensively from limited floral resources. This work will be published shortly as part of a broader review of the effects of pesticides on bees.
|
Masumi Gudka, a Kenyan Master’s student (at the University of Cape Town/Percy FitzPatrick Institute of African Ornithology), is investigating the exposure of African fish eagles (Haliaeetus vocifer) to organochlorine compounds in Lake Naivasha and Lake Baringo under the supervision of Dr Munir Virani and Dr Rob Simmons. As top predators and a revenue-generating tourist attraction, the fish eagle is a highly emblematic species that provides important ecological and economic services to the rift valley lakes, where they are found in high densities. The African population appears to have decreased over the last two decades at both lakes. Increased agriculture in and around the lakes and subsequent chemical runoff into the lake waters could be contributing to this decline. The objective of the study is to screen water, sediment, fish and fish eagle blood sampled for pesticides and compare chemical residues with areas in close proximity to flower farms. This study is important because few have considered the impact of organochlorine residues in Kenyan freshwater lakes and their supported biodiversity. Historical databases of OC residues for purposes of comparison are therefore lacking. Information on best practice sample collection and preservation are available but in tropical climates preserving sample integrity is tricky and difficult, especially without the use of on-site laboratories and facilities.
Conclusions
The JKUAT laboratory will liaise with the relevant laboratories and researchers to remain aware of developments in the conservation and analytical community. Additionally, links between FASTA and PACN are ensuring that chemists across the entire African continent are collaborating and facilitating collaboration with UK scientists. Given the wealth of experience represented, and the dedication shown, we have no doubt that we will be able to develop and implement a highly efficient and successful monitoring system in the years ahead. Colleagues wishing to learn more or contribute to any of the initiatives described in this article or to offer analytical expertise and other means of support are invited to contact Steve Lancaster and Ngaio Richards.
References
1. S. Lancaster, Mass Matters, 2006, 51,11.
2. S. Crossland, Mass Matters, 2006, 52, 6.
3. N. Richards and S. Lancaster, Mass Matters, 2007, 54, 6.
4. J. L. Oaks, M. Gilbert, M. Z. Virani, R. T. Watson, C. U. Meteyer, B. A. Rideout, H. L Shivaprasad, S. Ahmed, M. J. I. Chaudhry, M. Arshad, S. Mahmood, A. Ali, and A. A. Khan, Nature, 2004, 427, 630-633.
5. N. Richards, Chemistry & Industry, February 28th, 2008.
6. C. O’Driscoll, Chemistry & Industry, January 28th, 2008.
7. M. Odino and D. L. Ogada, Report to the Bird Committee of Nature Kenya, 2008.
8. N. L. Richards, G. Cooke, V. Simpson, S. Hall, N. M. Harrison and K. S. Scott, European Journal of Wildlife Research, 2011, DOI 10.1007/s10344-011-0513-2.
9. N. Richards S. Hall, K. Scott and N. Harrison, Environmental Pollution, 2011, 159, 1446.
10. P. O. Otieno, J. O. Lalah, M. Z. Virani, I. O. Jondiko and K.-W. Schramm, Journal of Environmental Science and Health, 2010, 45, 137.
11. P. O. Otieno, J. O. Lalah, M. Z. Virani, I. O. Jondiko and K.-W. Schramm, Bulletin of Environmental Contamination and Toxicology, 2010, 84, 536.
12. M. Z. Virani, C. Kendall, P. Njoroge and S. Thomsett, Biological Conservation, 2011, 144, 746.
13. D. L. Ogada, M. E. Torchin, V. O. Ezenwa and M. Kinnaird, Conservation Biology, in press.
14. D. J. Martins, Generalised protocol for sampling insects from carcasses in East Africa. Insect Committee of Nature Kenya – The East Africa Natural History Society. Nairobi, Kenya, 2010, Informally distributed to stakeholders
15. Martins, D. J. Differences in Odonata abundance and diversity in pesticide-fished, traditionally-fished and protected areas in Lake Victoria, Eastern Africa (Anisoptera). Odonatologica, 2009, 38, 247.
Authors*
Steven Lancaster (1), Ngaio L. Richards (1,2,3), Anthony Gachanja (1,4), Darcy Ogada (5), Masumi Gudka (6), Joseph Lalah (7), Peter Otieno (8), Munir Z. Virani (5,9), Dino J. Martins (10,11)
FOOTNOTES
1. The Investigative Chemistry Research Group is currently assessing the presence of NSAIDs in indicator species in the aquatic and terrestrial environment, see references 8 and 9 .
2. Ngaio Richards was also in Kenya to conduct research and interviews for a book she is editing on the global repercussions of the carbamate insecticide, Carbofuran, on wildlife populations. The royalties of this book will be placed in a research fund to further the analytical and conservation work underway, in partnership with JKUAT and FASTA.
WEB LINK
http://www.chemistry.manchester.ac.uk/groups/pob/fasta/
Conclusions
The JKUAT laboratory will liaise with the relevant laboratories and researchers to remain aware of developments in the conservation and analytical community. Additionally, links between FASTA and PACN are ensuring that chemists across the entire African continent are collaborating and facilitating collaboration with UK scientists. Given the wealth of experience represented, and the dedication shown, we have no doubt that we will be able to develop and implement a highly efficient and successful monitoring system in the years ahead. Colleagues wishing to learn more or contribute to any of the initiatives described in this article or to offer analytical expertise and other means of support are invited to contact Steve Lancaster and Ngaio Richards.
References
1. S. Lancaster, Mass Matters, 2006, 51,11.
2. S. Crossland, Mass Matters, 2006, 52, 6.
3. N. Richards and S. Lancaster, Mass Matters, 2007, 54, 6.
4. J. L. Oaks, M. Gilbert, M. Z. Virani, R. T. Watson, C. U. Meteyer, B. A. Rideout, H. L Shivaprasad, S. Ahmed, M. J. I. Chaudhry, M. Arshad, S. Mahmood, A. Ali, and A. A. Khan, Nature, 2004, 427, 630-633.
5. N. Richards, Chemistry & Industry, February 28th, 2008.
6. C. O’Driscoll, Chemistry & Industry, January 28th, 2008.
7. M. Odino and D. L. Ogada, Report to the Bird Committee of Nature Kenya, 2008.
8. N. L. Richards, G. Cooke, V. Simpson, S. Hall, N. M. Harrison and K. S. Scott, European Journal of Wildlife Research, 2011, DOI 10.1007/s10344-011-0513-2.
9. N. Richards S. Hall, K. Scott and N. Harrison, Environmental Pollution, 2011, 159, 1446.
10. P. O. Otieno, J. O. Lalah, M. Z. Virani, I. O. Jondiko and K.-W. Schramm, Journal of Environmental Science and Health, 2010, 45, 137.
11. P. O. Otieno, J. O. Lalah, M. Z. Virani, I. O. Jondiko and K.-W. Schramm, Bulletin of Environmental Contamination and Toxicology, 2010, 84, 536.
12. M. Z. Virani, C. Kendall, P. Njoroge and S. Thomsett, Biological Conservation, 2011, 144, 746.
13. D. L. Ogada, M. E. Torchin, V. O. Ezenwa and M. Kinnaird, Conservation Biology, in press.
14. D. J. Martins, Generalised protocol for sampling insects from carcasses in East Africa. Insect Committee of Nature Kenya – The East Africa Natural History Society. Nairobi, Kenya, 2010, Informally distributed to stakeholders
15. Martins, D. J. Differences in Odonata abundance and diversity in pesticide-fished, traditionally-fished and protected areas in Lake Victoria, Eastern Africa (Anisoptera). Odonatologica, 2009, 38, 247.
Authors*
Steven Lancaster (1), Ngaio L. Richards (1,2,3), Anthony Gachanja (1,4), Darcy Ogada (5), Masumi Gudka (6), Joseph Lalah (7), Peter Otieno (8), Munir Z. Virani (5,9), Dino J. Martins (10,11)
- Foundation for Analytical Science & Technology in Africa, St Ives, Cambridge, UK, PE27 3YN
- Working Dogs for Conservation, 52 Eustis Road, Three Forks, Montana, USA 59752
- Investigative Chemistry Research Group, Department of Life Sciences, Anglia Ruskin University, East Road, Cambridge, UK CB1 1PT
- Chemistry Department, Jomo Kenyatta University of Agriculture & Technology, P.O. Box 62000, 00200 Nairobi, Kenya
- The Peregrine Fund, 5668 West Flying Hawk Lane, Boise, Idaho 83709, USA
- The Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, Cape Town, South Africa
- Department of Chemical Sciences and Technology, School of Applied Sciences and Technology, Kenya Polytechnic University College, P.O. Box 52428-00200 City Square, Nairobi, Kenya
- Department of Chemistry, Maseno University, P.O Box 333, 40105, Maseno, Kenya
- Ornithology Section, Department of Zoology, National Museums of Kenya, P.O. Box 40658-00100, Nairobi, Kenya
- Insect Committee of Nature Kenya, National Museums of Kenya
- Turkana Basin Institute – Stonybrook University, N507 Social and Behavioural Sciences, Stony Brook, New York, USA 11794-4364
FOOTNOTES
1. The Investigative Chemistry Research Group is currently assessing the presence of NSAIDs in indicator species in the aquatic and terrestrial environment, see references 8 and 9 .
2. Ngaio Richards was also in Kenya to conduct research and interviews for a book she is editing on the global repercussions of the carbamate insecticide, Carbofuran, on wildlife populations. The royalties of this book will be placed in a research fund to further the analytical and conservation work underway, in partnership with JKUAT and FASTA.
WEB LINK
http://www.chemistry.manchester.ac.uk/groups/pob/fasta/