Emerging Organic Contaminants
Jamie Harrower, PhD Researcher
Glasgow Caledonian University/James Hutton Institute
[email protected]
ECG Bulletin February 2018
Glasgow Caledonian University/James Hutton Institute
[email protected]
ECG Bulletin February 2018
Emerging Organic Contaminants (EOCs) have attracted the interest of the international science community, with numerous studies demonstrating the presence of EOCs in significant concentrations within the environment. The term Emerging Organic Contaminants relates to newly synthesised compounds that are recognised as hazardous to wildlife, ecosystems, and potentially humans. Advances in analytical technologies have created instruments capable of detecting such compounds at low concentrations, even within complex matrices.
Until the early 1990s, non-polar persistent organic pollutants (POPs) and heavy metals were the main focus of intensive monitoring programmes; these include poly-aromatic hydrocarbons (PAHs) such as fluoranthene and benzo[a]pyrene and hydrocarbon solvents such as toluene and xylene (1). Today, these compounds are less relevant in industrialised countries thanks to drastic emission reductions. However, because of their extensive historical use and presence within industry, significant levels persist within the environment.
Until the early 1990s, non-polar persistent organic pollutants (POPs) and heavy metals were the main focus of intensive monitoring programmes; these include poly-aromatic hydrocarbons (PAHs) such as fluoranthene and benzo[a]pyrene and hydrocarbon solvents such as toluene and xylene (1). Today, these compounds are less relevant in industrialised countries thanks to drastic emission reductions. However, because of their extensive historical use and presence within industry, significant levels persist within the environment.
Fate of EOCs
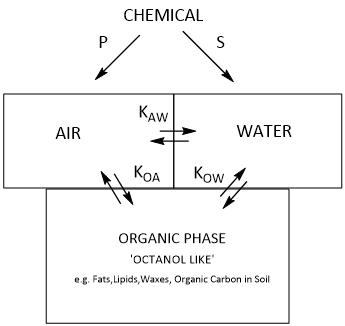
Emerging organic contaminants are generally hydrophobic by nature, with varying degrees of polarity, and varying physicochemical properties (pka, water solubility, partition coefficient and vapour pressure). Triclosan, Carbamazepine and Diclofenac are typical EOCs (with vapour pressure values of 6.9 X 10-5 Pa, 1.17 x 10-5 and 2.91 x 10-4 Pa respectively) and can be present in different physical states under particular environmental conditions.The fate and transport of EOCs within the environment is heavily dependent on their physicochemical properties and the surrounding environmental conditions, such as pH of the aqueous phase and soil composition. EOCs can also degrade or transform into metabolites, which in turn are able to partition and migrate into other phases in the environment, making it challenging to predict their behaviour. The transfer of chemicals between two or more environmental compartments can be described by equilibrium partitioning, which takes place between adjacent phases such as between a solid and a liquid (dissolution) or a liquid and a gas (volatilisation) (2). This net transfer of an organic compound between phases is limited by equilibrium constraints and can be quantified according to partition coefficients (see Figure 1).
|
Several partition coefficients are essential for understanding chemical transfer in the environment as well as the fundamental properties of vapour pressure (P) and solubility of a compound (S). Important partition coefficients that determine a chemical’s fate include air/water (Kaw), n-octanol/water (Kow) and n-octanol/air (Koa). The aqueous solubility and vapour pressure (effectively the solubility of a chemical in air) of a compound determine the partitioning of a chemical from pure liquid or solid into air and water.
Sources and classes of EOCsEOCs are found in pharmaceutical and personal care products, pesticides, veterinary products, industrial compounds/by-products, food additives, and engineered nano-materials. The rise in EOCs in recent years has resulted in a swift response from the science community. |
Organic contaminants can enter the environment through landfill sites, animal and domestic waste, hospital and industrial effluent, septic tanks and waste water treatment plants (WWTPs). In developed countries, the main contributor of EOCs to the environment is the discharge of treated wastewater from WWTPs (3). Studies have been conducted throughout Europe showing the presence of 125 different polar organic compounds within the effluent of 90 WWTPs (4).
|
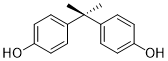
The impact of these contaminants on human health is often unknown, however some organic compounds have been classed as Endocrine Disrupting Compounds (EDCs) and have been detected in sewage sludge and sewage sludge-amended soils. EDCs can also cause adverse effects on wildlife and the ecological system (5). A well-known EDC is Bisphenol A (see Figure 2), which has recently been placed on the list as a substance of very high concern (European Chemicals Agency).
|
Wastewater treatment processes
Technology at WWTPs is continually improving and developing to ensure the safe and effective treatment of raw sewage. The activated sludge process is the most extensively implemented secondary wastewater treatment process and is effective at removing carbonaceous material and certain nutrients (6). Many drugs of anthropogenic origin are removed through biological degradation and sorption onto solids. However, their varying degrees of sorption and biodegradation make enhanced EOC removal rates at WWTPs very different. Also, many pharmaceutical and organic compounds have low vapour pressures, which restricts them from being removed by volatilisation. It should be noted that for several processes, removing the pollutant from water simply transfers it to solid waste or to the atmosphere.To further enhance EOC removal following the secondary waste effluent treatment process, tertiary processes have been tested, such as biofiltration (sand or trickling filters), chemical oxidation and adsorption by activated carbon (6). However, once again, the efficiency of the removal processes is highly compound-specific.
Sample preparation and analytical procedures
Analytical techniques such as Gas Chromatography- Mass Spectrometry and Liquid Chromatography-Mass Spectrometry are the most commonly applied techniques for detecting compounds within environmental matrices. Many studies have used the triple quadrupole mass detector (MS/MS), which offers the ability for enhanced mass fragment analysis of micropollutants. Sample pre-treatment is crucial when analysing trace contaminants at concentrations in the mgL-1 or µgL-1 scale in environmental samples, but is particularly important for extracting solid (soil) mixtures. Accelerated solvent extraction (ASE) provides a selective and efficient extraction by drawing solvent mixtures through a metal sample cell at high pressure (7). Further sample clean up using solid phase extraction (SPE) may be required after extraction is complete, and depending on what analytical technique is employed for analysis, a sample derivatisation step may also be needed.The release of EOCs to the environment will continue as new consumer products are constantly being brought to market. Therefore, the challenge to remove and prevent EOCs from entering the environment remains an international task. Legislation to control and minimise the release of dangerous chemicals into the environment, such as the Water Framework Directive (WFD, Directive 2000/60/EC) aim to meet certain environmental quality standards in all surface and ground waters (rivers, lakes, transitional waters and coasts), with a focus on protecting ecology and wildlife. The directive 2013/39/EU, amending the Environmental Quality Standards Directive 2008/105/EC under the European WFD, has introduced a new “Watch list” monitoring mechanism to collect high-quality EU-wide monitoring data of potentially polluting substances in the aquatic environment. To date, there are numerous other pharmaceutical compounds detected within the environment, which are continually being considered for the "Watch list".
References
1. Petrovic, M. et al., Environmental Chemistry 5, 1-35 (2008)
2. Harrison, R. M. Principles of Environmental Chemistry. London, UK; Royal Society of Chemistry Publishing. (2007)
3. Petrie, B. et al., Water research 72, 3-27 (2015).
4. Loos, R. et al., Water Research 47, 6475- 6487 (2013).
5. Rhind, S.M. et al., Environment Pollution 181, 262-270 (2013)
6. Petrie, B. et al,. Trends in Analytical Chemistry, 49: 145-159 (2013)
1. Petrovic, M. et al., Environmental Chemistry 5, 1-35 (2008)
2. Harrison, R. M. Principles of Environmental Chemistry. London, UK; Royal Society of Chemistry Publishing. (2007)
3. Petrie, B. et al., Water research 72, 3-27 (2015).
4. Loos, R. et al., Water Research 47, 6475- 6487 (2013).
5. Rhind, S.M. et al., Environment Pollution 181, 262-270 (2013)
6. Petrie, B. et al,. Trends in Analytical Chemistry, 49: 145-159 (2013)