ECG Atmospheric and Environmental Chemistry Forum 2013
Meeting report edited by William Bloss and Steven Ball
ECG Committee Member
ECG Bulletin February 2013
ECG Committee Member
ECG Bulletin February 2013
The Environmental Chemistry Group held its third Forum for early career researchers in atmospheric and environmental chemistry at the Department of Chemistry, Leicester University on Monday, June 25th, 2012. The event was attended by PhD students from numerous British universities. All delegates gave either a 15 minute talk or a poster presentation on their own research. Delegates had ample time to network and exchange expertise and opinions during lunch, the poster session and the careers discussion.
The Forum’s invited keynote lecture Air quality: turning science into policy” was given by Professor Paul Monks (Leicester University), who provided an informative insight into the mechanisms through which scientific understanding informs legislation and policy. This subject was particularly appropriate for an ECG Forum because “policy” is a vitally important end-use of research knowledge, yet one that is typically beyond the experience of researchers at the early stages of their careers.
After the keynote lecture, there was a wide-ranging careers discussion facilitated by an “expert panel” comprising Prof. Mat Evans (York University), Dr Helen Walker (AEA Technology) and Charlotte Ashley-Roberts (RSC careers adviser). We are grateful to our experts for contributing their time, experience and enthusiasm. We acknowledge an award from the RSC Travel Grant Scheme that enabled the organisers to offer student bursaries to attend the Forum, and sponsorship of some of the Forum’s costs by NERC’s National Centre for Atmospheric Research.
Below we present a selection of abstracts from the Forum delegates’ talks and posters. The abstracts were provided by the forum delegates and compiled and edited by Stephen Ball and Bill Bloss. Additionally Prof. Monks has written a short summary article based on his keynote lecture; his article appears on page 17 of this edition of the ECG Bulletin.
The Forum’s invited keynote lecture Air quality: turning science into policy” was given by Professor Paul Monks (Leicester University), who provided an informative insight into the mechanisms through which scientific understanding informs legislation and policy. This subject was particularly appropriate for an ECG Forum because “policy” is a vitally important end-use of research knowledge, yet one that is typically beyond the experience of researchers at the early stages of their careers.
After the keynote lecture, there was a wide-ranging careers discussion facilitated by an “expert panel” comprising Prof. Mat Evans (York University), Dr Helen Walker (AEA Technology) and Charlotte Ashley-Roberts (RSC careers adviser). We are grateful to our experts for contributing their time, experience and enthusiasm. We acknowledge an award from the RSC Travel Grant Scheme that enabled the organisers to offer student bursaries to attend the Forum, and sponsorship of some of the Forum’s costs by NERC’s National Centre for Atmospheric Research.
Below we present a selection of abstracts from the Forum delegates’ talks and posters. The abstracts were provided by the forum delegates and compiled and edited by Stephen Ball and Bill Bloss. Additionally Prof. Monks has written a short summary article based on his keynote lecture; his article appears on page 17 of this edition of the ECG Bulletin.
Section 1: Field studies and ambient air measurements
1. Investigating Air Pollution in London. Rachel Holmes, Richard Lidster, Jacqueline F. Hamilton, James D. Lee and James R. Hopkins,
Department of Chemistry, University of York
The majority of the World’s population lives in polluted urbanized areas. Poor air quality is shortening the life expectancy of people in the UK by an average of 7-8 months and costs the UK economy around £20 billion per year.1 Despite this, our understanding of the atmospheric processing of pollutants in urban environments and their effect on air quality remains poor. The Clean Air for London (ClearfLo) project aims to investigate boundary layer pollution across London and thus improve the prediction capability of air quality models. Numerous previous studies in urban environments show discrepancies between the observed concentrations of short-lived atmospheric radicals and values calculated using explicit chemical models. One possibility is that copious reactive sinks for radicals are missing from the models, and one candidate is unmeasured volatile organic compounds (VOCs) especially larger aromatic species.
To obtain measurements on (as close to possible) the full suite of atmospheric volatile organic compounds, we have developed a method using comprehensive two dimensional gas chromatography coupled to a flame ionisation detector (GCxGC-FID). This is a high resolution method, with increased separation power and improved peak capacity when compared to many single column systems.2 Air samples were collected at hourly intervals during the first field campaign of the ClearfLo project in Jan/Feb 2012. These air samples were analysed for C4 to C12 VOCs. The GCxGC-FID system has separated over 80 species (of which 22 have so far been identified), including oxygenates, aromatics, and saturated and unsaturated aliphatics. Including these extra VOCs should improve the prediction capability of air quality models.
1. House of Commons Environmental Audit Committee, “Air Quality: A follow-up report”, Ninth Report of Session 2010-12, Volume 1 (and references within).
2. Lidster, R. T., Hamilton, J. F. and Lewis, A. C., Journal of Separation Science, 34, 812-821, 2011.
Department of Chemistry, University of York
The majority of the World’s population lives in polluted urbanized areas. Poor air quality is shortening the life expectancy of people in the UK by an average of 7-8 months and costs the UK economy around £20 billion per year.1 Despite this, our understanding of the atmospheric processing of pollutants in urban environments and their effect on air quality remains poor. The Clean Air for London (ClearfLo) project aims to investigate boundary layer pollution across London and thus improve the prediction capability of air quality models. Numerous previous studies in urban environments show discrepancies between the observed concentrations of short-lived atmospheric radicals and values calculated using explicit chemical models. One possibility is that copious reactive sinks for radicals are missing from the models, and one candidate is unmeasured volatile organic compounds (VOCs) especially larger aromatic species.
To obtain measurements on (as close to possible) the full suite of atmospheric volatile organic compounds, we have developed a method using comprehensive two dimensional gas chromatography coupled to a flame ionisation detector (GCxGC-FID). This is a high resolution method, with increased separation power and improved peak capacity when compared to many single column systems.2 Air samples were collected at hourly intervals during the first field campaign of the ClearfLo project in Jan/Feb 2012. These air samples were analysed for C4 to C12 VOCs. The GCxGC-FID system has separated over 80 species (of which 22 have so far been identified), including oxygenates, aromatics, and saturated and unsaturated aliphatics. Including these extra VOCs should improve the prediction capability of air quality models.
1. House of Commons Environmental Audit Committee, “Air Quality: A follow-up report”, Ninth Report of Session 2010-12, Volume 1 (and references within).
2. Lidster, R. T., Hamilton, J. F. and Lewis, A. C., Journal of Separation Science, 34, 812-821, 2011.
2. Field Measurements and Modelling Studies of Radicals during the ClearfLo Campaign
Noel Clancy and Dwayne E. Heard, School of Chemistry, University of Leeds
Noel Clancy and Dwayne E. Heard, School of Chemistry, University of Leeds
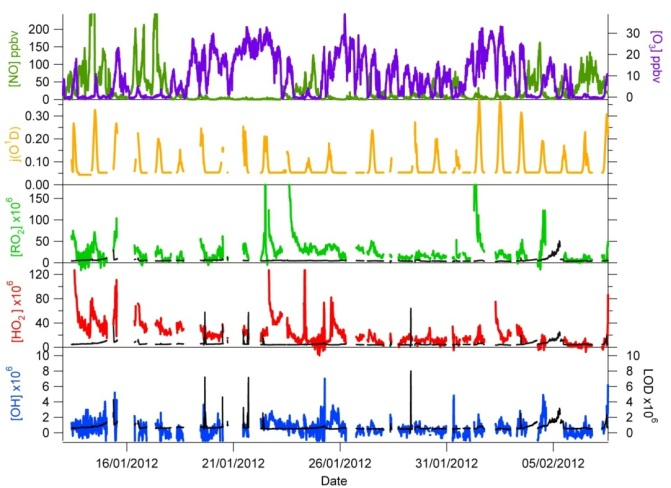
Urban air pollution endured in London and other large cities has adverse effects on human health. Current understanding of the processes responsible for London’s poor air quality is incomplete, in large part because the photo-oxidation chemistry occurring in urban atmospheres is highly complex. This project aims to improve the accuracy with which atmospheric chemistry is parameterised within climate and air quality models by making detailed field measurements of atmospheric radicals (OH, HO2 and RO2) in the London environment. Radicals initiate and propagate the oxidation cycles that remove harmful trace gases (e.g. CO, benzene) from the atmosphere.
3. Isoprene in the Remote Marine Boundary Layer
Sina C. Hackenberg, Lucy J. Carpenter and Alastair C. Lewis, Department of Chemistry, University of York
Oceanic organic carbon aerosol has been suggested to have a significant effect on climate through modification of the microphysical properties of low-level marine stratocumulus clouds.1 Isoprene and monoterpenes (reactive volatile organic compounds emitted by phytoplankton) have been proposed to increase cloud albedo in the region of phytoplankton blooms due to the formation of such secondary organic aerosol [1,2].
Several studies have shown that isoprene and monoterpene emissions vary depending on light, temperature, phytoplankton abundance and functional type.1 However, the importance of isoprene and monoterpenes in the remote marine boundary layer is still unclear due to a lack of observations of its seasonal and diurnal variability, and the resulting uncertainties in model estimates of global oceanic emission fluxes.1 Using existing and new measurements of isoprene and monoterpenes in marine air and seawater, this project investigates the relationships with meteorological and biological parameters in order to improve our understanding of the mechanisms and processes that control marine terpene emissions. The ultimate aim is to calculate new estimates of sea-air fluxes of these compounds.
1. Shaw, S. L., Gantt, B. and Meskhidze, N., Advances in Meteorology, doi:10.1155/2010/408696, 2010.
2. Meskhidze, N. and Nenes, A., Science, 314, 1419, 2006.
4. Speciated NOy Measurements in the Remote Tropical Troposphere at the Cape Verde Atmospheric Observatory (CVAO)
William D. Manning, James D. Lee and Lucy J. Carpenter, Department of Chemistry, University of York
Sina C. Hackenberg, Lucy J. Carpenter and Alastair C. Lewis, Department of Chemistry, University of York
Oceanic organic carbon aerosol has been suggested to have a significant effect on climate through modification of the microphysical properties of low-level marine stratocumulus clouds.1 Isoprene and monoterpenes (reactive volatile organic compounds emitted by phytoplankton) have been proposed to increase cloud albedo in the region of phytoplankton blooms due to the formation of such secondary organic aerosol [1,2].
Several studies have shown that isoprene and monoterpene emissions vary depending on light, temperature, phytoplankton abundance and functional type.1 However, the importance of isoprene and monoterpenes in the remote marine boundary layer is still unclear due to a lack of observations of its seasonal and diurnal variability, and the resulting uncertainties in model estimates of global oceanic emission fluxes.1 Using existing and new measurements of isoprene and monoterpenes in marine air and seawater, this project investigates the relationships with meteorological and biological parameters in order to improve our understanding of the mechanisms and processes that control marine terpene emissions. The ultimate aim is to calculate new estimates of sea-air fluxes of these compounds.
1. Shaw, S. L., Gantt, B. and Meskhidze, N., Advances in Meteorology, doi:10.1155/2010/408696, 2010.
2. Meskhidze, N. and Nenes, A., Science, 314, 1419, 2006.
4. Speciated NOy Measurements in the Remote Tropical Troposphere at the Cape Verde Atmospheric Observatory (CVAO)
William D. Manning, James D. Lee and Lucy J. Carpenter, Department of Chemistry, University of York
5. Determination of the Atmospheric Loss Rates of NO3 and N2O5 using Observational Data from the RONOCO Campaign
M. McLeod, B. Ouyang and R. L. Jones, Department of Chemistry, University of Cambridge
During the day, the OH radical acts as the dominant species driving oxidation chemistry in the troposphere (the lowermost atmospheric layer). But because there can be no photochemical production of OH radicals at night, another oxidant species, the nitrate radical (NO3), takes over the role of initiating night-time oxidative reactions [1]. NO3 is in thermal equilibrium with N2O5 and both species can establish significant concentrations at night. As well as potentially affecting ozone budgets via their night-time chemistry, physical and/or chemical removal of NO3 and N2O5 also acts as an important sink for removing NOx from the atmosphere [2]. The aim of the RONOCO (ROle of Night-time chemistry in controlling the Oxidising Capacity of the atmOsphere) campaign was to quantify the processes influencing night-time chemistry over the UK and near-continental Europe using a combination of airborne measurements and atmospheric modelling on different scales.
The RONOCO consortium comprised research groups from the Universities of Cambridge, East Anglia, Leicester, Leeds, Manchester and York. Measurements, including NO3, N2O5 , NOx, O3, CO, aerosol, HNO3, peroxy alkyl nitrates and volatile organic compounds, were taken from on-board the FAAM (Facility for Airborne Atmospheric Measurements) BAe-146 research aircraft during flights around the UK in July-August 2010 and January 2011. A total of ninety flying hours were completed. Here we present NO3, N2O5 and NO2 measurements made with a new three channel broadband cavity enhanced absorption spectroscopy instrument [3]. Figure 3 shows an example of measurements taken on one flight during the January 2011 campaign. In this presentation, we explore different methods by which the rate constants for direct chemical loss of NO3 and the aerosol uptake coefficient of N2O5 can be derived. We also investigate how to appropriately partition the total sink of NO3 and N2O5 between the individual loss processes under the different chemical environments experienced during the RONOCO campaign.
During the day, the OH radical acts as the dominant species driving oxidation chemistry in the troposphere (the lowermost atmospheric layer). But because there can be no photochemical production of OH radicals at night, another oxidant species, the nitrate radical (NO3), takes over the role of initiating night-time oxidative reactions [1]. NO3 is in thermal equilibrium with N2O5 and both species can establish significant concentrations at night. As well as potentially affecting ozone budgets via their night-time chemistry, physical and/or chemical removal of NO3 and N2O5 also acts as an important sink for removing NOx from the atmosphere [2]. The aim of the RONOCO (ROle of Night-time chemistry in controlling the Oxidising Capacity of the atmOsphere) campaign was to quantify the processes influencing night-time chemistry over the UK and near-continental Europe using a combination of airborne measurements and atmospheric modelling on different scales.
The RONOCO consortium comprised research groups from the Universities of Cambridge, East Anglia, Leicester, Leeds, Manchester and York. Measurements, including NO3, N2O5 , NOx, O3, CO, aerosol, HNO3, peroxy alkyl nitrates and volatile organic compounds, were taken from on-board the FAAM (Facility for Airborne Atmospheric Measurements) BAe-146 research aircraft during flights around the UK in July-August 2010 and January 2011. A total of ninety flying hours were completed. Here we present NO3, N2O5 and NO2 measurements made with a new three channel broadband cavity enhanced absorption spectroscopy instrument [3]. Figure 3 shows an example of measurements taken on one flight during the January 2011 campaign. In this presentation, we explore different methods by which the rate constants for direct chemical loss of NO3 and the aerosol uptake coefficient of N2O5 can be derived. We also investigate how to appropriately partition the total sink of NO3 and N2O5 between the individual loss processes under the different chemical environments experienced during the RONOCO campaign.
1. Brown, S. S. and Stutz, J., Chemical Society Reviews, 41, 6405, 2012.
2. Chang, W. L., Bhave, P. V., Brown, S. S., et al., Aerosol Science and Technology, 45, 665, 2011.
3. Kennedy, O. J., Ouyang, B., Langridge, J. M., Daniels, M. J. S., Bauguitte, S., Freshwater, R., McLeod, M. W., Ironmonger, C., Sendall, J., Norris, O., Nightingale, R., Ball, S. M., Jones, R. L., Atmospheric Measurement Techniques, 4,1759, 2011.
2. Chang, W. L., Bhave, P. V., Brown, S. S., et al., Aerosol Science and Technology, 45, 665, 2011.
3. Kennedy, O. J., Ouyang, B., Langridge, J. M., Daniels, M. J. S., Bauguitte, S., Freshwater, R., McLeod, M. W., Ironmonger, C., Sendall, J., Norris, O., Nightingale, R., Ball, S. M., Jones, R. L., Atmospheric Measurement Techniques, 4,1759, 2011.
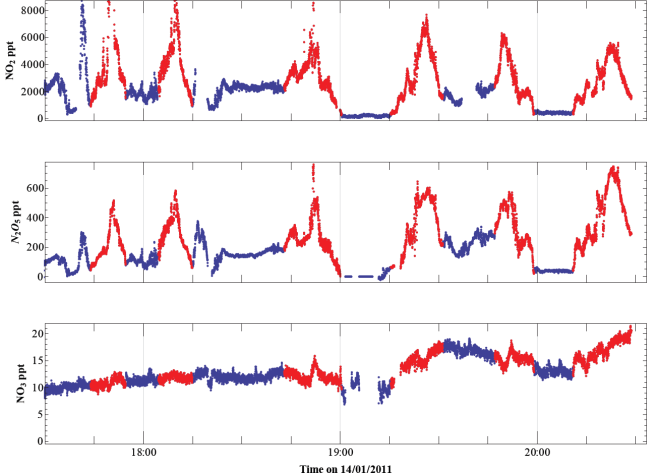
Figure 3: Time series of NO2, N2O5 and NO3 concentrations recorded on a winter flight over the North Sea, starting 90 minutes after sunset. Highlighted in red are several transects across the same pollution plume originating from the Liverpool and Manchester urban areas. Each transect is further down-stream of the source; the N2O5 concentrations increase on each transect, showing how night-time chemistry within the plume evolves, converting NO2 into N2O5.
6. Comparison of Reactive Gas Emissions from Bioenergy and Arable Crops
E. C. Morrison (1,2), M. R. Heal (1) and J. Drewer (2)
1 School of Chemistry, University of Edinburgh
2 Centre for Ecology and Hydrology, Penicuik, Edinburgh
E. C. Morrison (1,2), M. R. Heal (1) and J. Drewer (2)
1 School of Chemistry, University of Edinburgh
2 Centre for Ecology and Hydrology, Penicuik, Edinburgh
Depletion of fossil fuel resources, pollution concerns and the challenge of energy security are driving the search for renewable energy sources. Around 7% of all UK arable land (~1.3 M ha) may be planted with bioenergy crops by 2020 in order to meet renewable energy and CO2 reduction targets. Although bioenergy crops are perceived as ‘carbon neutral’, changes in land use can have a wider impact on atmospheric composition than through their CO2 emissions alone. This study compares vegetation fluxes of methyl halides and volatile organic compounds (VOCs) from adjacent bioenergy and arable crops. Methyl halide gases impact on levels of stratospheric ozone, whilst VOCs affect atmospheric oxidising capacity and cause the formation of tropospheric ozone and secondary organic aerosols. There are very few previous measurements of these reactive gases from bioenergy crops.
All fluxes measured demonstrate a strong seasonal trend with higher emissions occurring during growing season and low to zero emissions over winter. A diurnal pattern of high emissions during the day and low to zero emissions at night is also seen. These are the first reported measurements of methyl halides from bioenergy crops. The highest fluxes for both CH3Br and CH3Cl were produced by oilseed rape. Standardised emission rates of isoprene from willow of 0.01-1 ng per gramme dry weight per hour have previously been reported, but our measurements indicate that the real figure could be much higher. Brassica species such as oilseed rape are known to be high emitters of CH3Br, as corroborated by these measurements; therefore extensive planting whether for food or fuel is likely to contribute to ozone layer destruction. High isoprene emissions from willow mean that increased planting, coupled with a warming climate, could have a considerable effect on atmospheric composition.
All fluxes measured demonstrate a strong seasonal trend with higher emissions occurring during growing season and low to zero emissions over winter. A diurnal pattern of high emissions during the day and low to zero emissions at night is also seen. These are the first reported measurements of methyl halides from bioenergy crops. The highest fluxes for both CH3Br and CH3Cl were produced by oilseed rape. Standardised emission rates of isoprene from willow of 0.01-1 ng per gramme dry weight per hour have previously been reported, but our measurements indicate that the real figure could be much higher. Brassica species such as oilseed rape are known to be high emitters of CH3Br, as corroborated by these measurements; therefore extensive planting whether for food or fuel is likely to contribute to ozone layer destruction. High isoprene emissions from willow mean that increased planting, coupled with a warming climate, could have a considerable effect on atmospheric composition.
Section 2: New and emerging instrumentation
7. Detection and Quantification of Glyoxal, Methylglyoxal and NO2 by BBCEAS
Thomas J. Adams, Mark J. S. Daniels and Stephen M. Ball, Department of Chemistry, University of Leicester
Glyoxal (CHOCHO) and methylglyoxal (CH3C(O)CHO) are common intermediates formed in the OH initiated photo-oxidation of a wide range of volatile organic compounds (VOCs) emitted from both anthropogenic and biogenic sources [1]. Such α-dicarbonyl compounds have been reported as potential precursors of secondary organic aerosol (SOA), and therefore may account for a proportion of the SOA currently missing from atmospheric models [2]. NO2 is emitted primarily through the burning of fuels and has a significant role in the chemistry of the urban air quality: the catalytic photochemical cycling of NOx (NO + NO2) is the major source of ozone in the troposphere. As well as being a respiratory irritant, ozone plays a significant role in the chemical processing of reactive trace gases in the lower atmosphere.
Broadband Cavity Enhanced Absorption Spectroscopy (BBCEAS) is a highly sensitive absorption spectroscopic technique that has been applied to detect and quantify trace gases in the atmosphere and in atmospheric simulation chambers [3]. Gases are sampled into the instrument and into an optical cavity constructed between two highly reflective mirrors. The effective path length of the absorption measurement is increased typically several thousand-fold through multiple reflections of light inside the cavity, thereby enabling target compounds to be quantified at sub-parts-per-billion concentrations. Molecules are identified by their absorption features at characteristic wavelengths and concentrations retrieved through fitting absorption features with reference absorption cross sections. Figure 4 shows an example of simultaneous glyoxal, methylglyoxal and NO2 measurements from recent experiments at the EUPHORE atmospheric chamber in Valencia (Spain) aimed at quantifying glyoxal and methylglyoxal yields from the photochemical oxidation of unsaturated volatile organic compounds.
Glyoxal (CHOCHO) and methylglyoxal (CH3C(O)CHO) are common intermediates formed in the OH initiated photo-oxidation of a wide range of volatile organic compounds (VOCs) emitted from both anthropogenic and biogenic sources [1]. Such α-dicarbonyl compounds have been reported as potential precursors of secondary organic aerosol (SOA), and therefore may account for a proportion of the SOA currently missing from atmospheric models [2]. NO2 is emitted primarily through the burning of fuels and has a significant role in the chemistry of the urban air quality: the catalytic photochemical cycling of NOx (NO + NO2) is the major source of ozone in the troposphere. As well as being a respiratory irritant, ozone plays a significant role in the chemical processing of reactive trace gases in the lower atmosphere.
Broadband Cavity Enhanced Absorption Spectroscopy (BBCEAS) is a highly sensitive absorption spectroscopic technique that has been applied to detect and quantify trace gases in the atmosphere and in atmospheric simulation chambers [3]. Gases are sampled into the instrument and into an optical cavity constructed between two highly reflective mirrors. The effective path length of the absorption measurement is increased typically several thousand-fold through multiple reflections of light inside the cavity, thereby enabling target compounds to be quantified at sub-parts-per-billion concentrations. Molecules are identified by their absorption features at characteristic wavelengths and concentrations retrieved through fitting absorption features with reference absorption cross sections. Figure 4 shows an example of simultaneous glyoxal, methylglyoxal and NO2 measurements from recent experiments at the EUPHORE atmospheric chamber in Valencia (Spain) aimed at quantifying glyoxal and methylglyoxal yields from the photochemical oxidation of unsaturated volatile organic compounds.
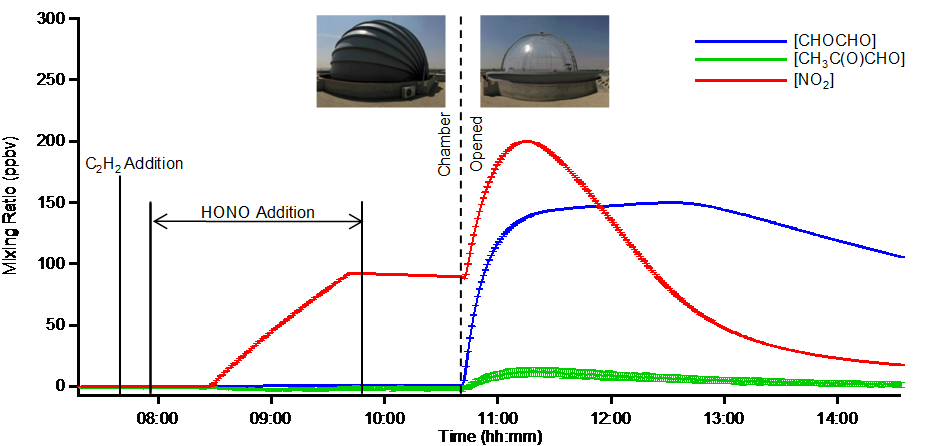
Figure 4: Time series of glyoxal, methylglyoxal and NO2 measured simultaneously by BBCEAS in a chamber study into the photo-chemical oxidation of acetylene. The addition of nitrous acid (HONO) acts both as a source of NOx (NO and NO2) and as the photolytic source of OH radicals when the chamber roof is opened at 10:45 in order to expose the gas mixture to light.
1. Calvert, J. G., Atkinson, R., Becker, K. H., Kamens, R. M., Seinfeld, J. H., Wallington, T. J., and Yarwood, G., The Mechanisms of Atmospheric Oxidation of Aromatic Hydrocarbons, Oxford Univ. Press, 2002.
2. Fu, T.-M., Jacob, D. J., Wittrock, F., Burrows, J. P., Vrekoussis, M., and Henze, D. K., Journal of Geophysical Research, 113, D15303, 2008
3. Ball. S. M., Langridge, J. M., and Jones, R. L., Chemical Physics Letters, 398, 68, 2004
8. Increased Sensitivity in Proton Transfer Reaction Mass Spectrometry by Incorporation of a Radio Frequency Ion Funnel
Shane Barber (1), Robert S. Blake (1) , Iain R. White (1), Paul S. Monks (1), Fraser Reich (2), Steve Mullock (2) and Andrew M. Ellis
1.Department of Chemistry, University of Leicester
2.Kore Technology Limited, Cambridgeshire Business Park, Ely, Cambridgeshire.
Proton Transfer Reaction-Time of Flight-Mass Spectrometry (PTR-ToF-MS) has proven itself a versatile technique capable of measuring a wide range of trace volatile organic compounds (VOCs) and oxygenated volatile organic compounds (oVOCs). Its applications are varied, ranging from both indoor and outdoor atmospheric chemistry to the medical and forensic sciences.1 In contrast to more conventional forms of mass spectrometry, PTR-ToF-MS captures data from all mass channels within a potentially complex gas mixture, simultaneously and in real time. This allows VOCs to be detected in situ, whether the measurements are performed in the laboratory, hospital or in the field.
2. Fu, T.-M., Jacob, D. J., Wittrock, F., Burrows, J. P., Vrekoussis, M., and Henze, D. K., Journal of Geophysical Research, 113, D15303, 2008
3. Ball. S. M., Langridge, J. M., and Jones, R. L., Chemical Physics Letters, 398, 68, 2004
8. Increased Sensitivity in Proton Transfer Reaction Mass Spectrometry by Incorporation of a Radio Frequency Ion Funnel
Shane Barber (1), Robert S. Blake (1) , Iain R. White (1), Paul S. Monks (1), Fraser Reich (2), Steve Mullock (2) and Andrew M. Ellis
1.Department of Chemistry, University of Leicester
2.Kore Technology Limited, Cambridgeshire Business Park, Ely, Cambridgeshire.
Proton Transfer Reaction-Time of Flight-Mass Spectrometry (PTR-ToF-MS) has proven itself a versatile technique capable of measuring a wide range of trace volatile organic compounds (VOCs) and oxygenated volatile organic compounds (oVOCs). Its applications are varied, ranging from both indoor and outdoor atmospheric chemistry to the medical and forensic sciences.1 In contrast to more conventional forms of mass spectrometry, PTR-ToF-MS captures data from all mass channels within a potentially complex gas mixture, simultaneously and in real time. This allows VOCs to be detected in situ, whether the measurements are performed in the laboratory, hospital or in the field.
|
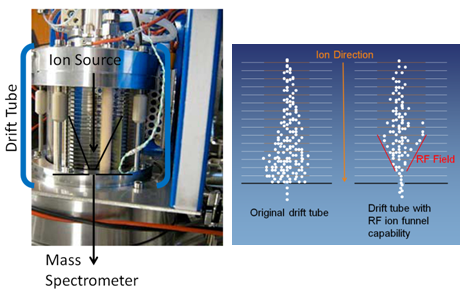
A drift tube that doubles as an ion funnel is demonstrated in proton transfer reaction mass spectrometry for the first time. The ion funnel enables a much higher proportion of (VOC+H)+ product ions to exit the drift tube and enter the mass spectrometer than would otherwise be the case (see Figure 5). A large increase in the detection sensitivity for volatile organic compounds of between one and two orders of magnitude is delivered, and has been characterized against dilution standards for a range of compounds. The improvements in both sensitivity and limit of detection allow this instrument to now detect a wider range of VOCs at a lower concentration. The instrument is currently being applied to quantify ambient VOC concentrations in order to help better understand atmospheric processes.
1. Barber S., Blake R. S., White I. R., Monks P. S., Reich F., Mullock S., Ellis A. M., Analytical Chemistry, 84, 5387, 2012.
|
9. Sensor Networks for Air Quality
V. B. Bright (1), M. I. Mead (1), O. A. M. Popoola (1), R. P. Baron (2), J. R. Saffell (2), G. B. Stewart (1) and R. L. Jones (1)
1 Department of Chemistry, University of Cambridge
2 Alphasense, Sensor Technology House, Great Notley
V. B. Bright (1), M. I. Mead (1), O. A. M. Popoola (1), R. P. Baron (2), J. R. Saffell (2), G. B. Stewart (1) and R. L. Jones (1)
1 Department of Chemistry, University of Cambridge
2 Alphasense, Sensor Technology House, Great Notley
|
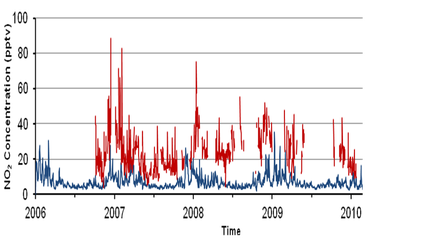
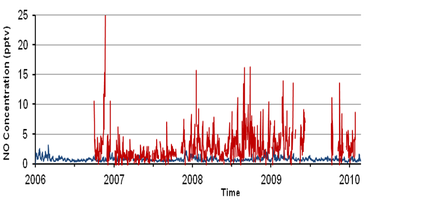
The ability of low-cost, portable devices that incorporate electrochemical sensors to measure gases such as CO, NO and NO2 at ambient concentrations has been demonstrated during deployments in urban areas including London, Valencia, Kuala Lumpur and Lagos. The sensors additionally include GPS (Global Positioning System) and GPRS (General Packet Radio Service) for positioning and data transmission, respectively. Laboratory tests carried out against gas standards at the parts-per-billion level have demonstrated the high sensitivity and linear response of electrochemical sensors to their respective target gases. Moreover, when such sensors are co-located with reference instruments in the field, they have shown a high level of agreement [1].
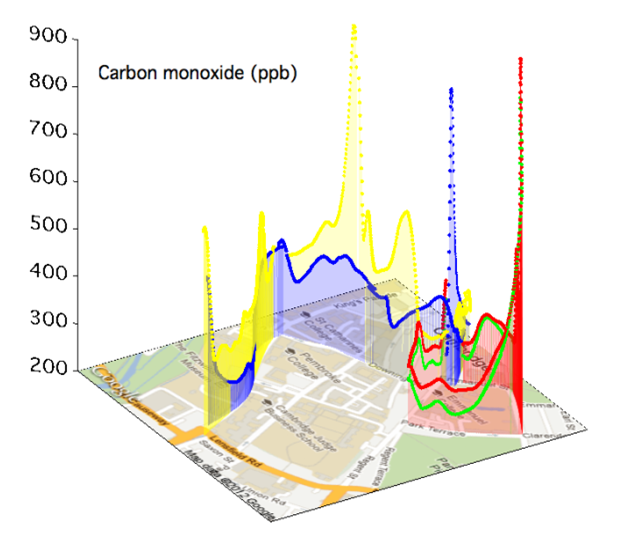
The degree of variability in pollutant levels, on both spatial and temporal scales, has been highlighted in various mobile sensor campaigns. An example of mobile carbon monoxide measurements around central Cambridge is shown in Figure 6. Such measurements also highlight the limitations of a sparsely populated static urban network that would fail to capture the highly variable concentration fields evident in Figure 6. Thus the technology outlined here was been extended to establish a dense, urban network of autonomous, static units capable of capturing data with high temporal resolution over a period of several months. |
The results of such deployments highlight the importance of meteorology, traffic and street canyon characteristics in determining the level of pollutants observed. In this presentation we show initial observations of NO, NO2, CO, CO2, SO2, O3, volatile organic compounds, size-speciated particulate matter and meteorological variables obtained using a high-density network of air quality sensors deployed in and around London Heathrow airport.
1. M. I. Mead, O.A.M. Popoola, G. B. Stewart, P. Landshoff, M. Calleja, M. Hayes, J. J. Baldovi, M. W. McLeod, T. F. Hodgson, J. Dicks, A. Lewis, J. Cohen, R. Baron, J. R. Saffell and R. L. Jones, submitted to Atmospheric Environment, 2012.
10. A New System for Measuring Ozone Production Rates
Hao Huang, William Bloss, Kate Faloon and Juan Najera, School of Geography, Earth and Environmental Science, University of Birmingham
10. A New System for Measuring Ozone Production Rates
Hao Huang, William Bloss, Kate Faloon and Juan Najera, School of Geography, Earth and Environmental Science, University of Birmingham
Ground level ozone is harmful to humans, vegetation and the environment, and is considered one of the principal air pollutants. In order to develop effective air quality policies to reduce ambient O3 concentrations, it is important to understand ozone production processes, and specifically the chemical ozone production rate, in order that this may be related to primary emissions. A direct ozone production rate measurement (“OPR method”) provides a new way to monitor in situ chemical ozone formation, complementing traditional modelling approaches [1]. The principle of the OPR system is to sample air into two parallel reactors; ambient chemical processing occurs as normal in one reactor (the sample reactor), whilst in the other the radical chemistry is suppressed (the reference reactor). The difference in ozone levels exiting the reactors, after correction for NOx photochemical steady state perturbation, determines the in situ ozone production rate. The design and initial deployment of a prototype OPR system is described, and future applications of the measurements are considered.
1. Cazorla, M., Brune, W. H., Ren, X., and Lefer, B., Atmospheric Chemistry and Physics, 12, 1203, 2012.
Section 3: Aerosol and particulate matter
11. Artificial Chemical Ageing of Ambient Atmospheric Aerosol
S. S. Al Kindi, R. M. Harrison and W. J. Bloss, School of Geography, Earth and Environmental Sciences, University of Birmingham
S. S. Al Kindi, R. M. Harrison and W. J. Bloss, School of Geography, Earth and Environmental Sciences, University of Birmingham
Atmospheric aerosol play critical roles in air quality, visibility, human health, regional and global climate, the ability to act as cloud condensation nuclei, precipitation events, atmospheric acid deposition, optical properties, atmospheric energy balance, and stratospheric ozone depletion [1]. Field measurements and air quality models have shown limitations in our understanding of the heterogeneous reactions and processing of aerosol-associated organic matter [2]. An experimental system for the artificial chemical ageing of atmospheric aerosol particles is described, and initial results from the processing of laboratory-generated oleic acid particles are presented. Within the apparatus, gas phase oxidant levels are maintained several orders of magnitude above ambient levels (e.g. [OH] = 0.5–2 x 1010 molecule cm-3 and [O3] = 1–50 ppm] such that atmospheric exposures equivalent to several days or weeks may be achieved in minutes in the laboratory (subject to limitations relating to particle mass transfer and diffusion).
|
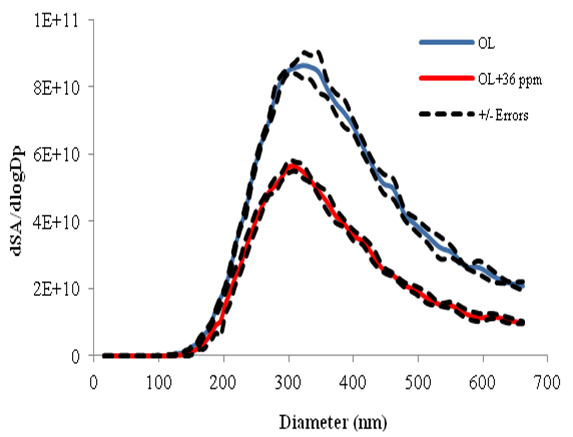
Figure 7 shows an example of the size distributions of oleic acid aerosol particles before and after processing by 36 parts per million of ozone. Measurements of the physical properties of processed aerosol particles are accomplished by a Scanning Mobility Particle Spectrometer (SMPS), while the chemical content of the particles is monitored by an Aerosol Time of Flight Mass Spectrometer (ATOFMS). The aim is to apply the technique to ambient atmospheric particles in order to assess their chemical evolution.
1. Kolb, C. E. and Worsnop, D. R., Annual Review of Physical Chemistry, 63, 471, 2012. 2. Simon, H. and Bhave, P. V., Environmental Science & Technology, 46, 331, 2012. |
12. Bioaccessibility of the Inhalable Fractions of Urban Road Dust
Andrew Brown (1), Sanja Potgieter-Vermaak (1,2), Judith Barret (1) and Rene Van Grieken (3)
1 Division of Chemistry & Environmental Science, Manchester Metropolitan University.
2 School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa.
3 Department of Chemistry, University of Antwerp, Belgium.
In vitro and animal toxicological studies have confirmed that the chemical composition of inhaled particles play a major role in their toxic, genotoxic and carcinogenic mechanisms, but the component-specific toxic effects are still not understood. Particle-bound airborne transition metals can also lead to the production of Reactive Oxygen Species in lung tissue; a special concern amongst particularly susceptible cohorts (children and elderly). Thus the bioaccessibility of the fine fraction aerosol is evidently of importance for public health.
Size-fractioned (<38, 38–63, 63–125 μm) road dust samples collected from one of the highest trafficked roads in the United Kingdom (and believed to be one of the busiest bus routes in Europe) were characterised for their bulk elemental composition with EDXRF, ICP-OES and ICP-MS, and their molecular composition with micro Raman spectroscopy (MRS). It was found that the fine fraction (<38 μm) had the highest Pb (238 ppm) and Cr (171 ppm) concentrations. Concentrations of both Pb and Cr decreased substantially in the largest particle size fractions (from 279 ppm in particles with diameters Dp <38 μm to 13 ppm in particles with Dp <1mm for lead; and from 171 ppm for Dp <38 μm to 91 ppm for Dp <1mm for chromium). The MRS data showed that the Cr was mostly present as lead chromate and therefore in the Cr(VI) oxidation state. Apart from rather alarmingly high concentrations of oxidative stressors (Cu, Fe, Mn) obtained from the elemental analysis, the carcinogenic potential of the respirable fraction is evident from the MRS data. These same fractions underwent in vitro testing to assess the mobility of toxic and carcinogenic components by leaching with artificial body fluids. Leachates were analysed for Cr, Cu, Zn, V, Pb, Mn, Cd, Fe, Ni, Al and As concentrations at time intervals from 1 hour to 8 weeks. In general, most of the elements leached in the ppb range and concentrations decreased with increasing particle size. Although the mobility in the artificial body fluids of the various elements varied, up to 19% Cr, 47% Pb and 87% Ni were released.
13. Improving the Modeling of Bioaerosol Dispersion from Green Waste Composting Facilities
Philippa Douglas1, Gillian Drew1, Rob Kinnersley2, Kerry Walsh2, Phil Longhurst1 and Sean Tyrrel1
1.Centre for Energy and Resource Technology, School of Applied Sciences, Cranfield University
2.Environment Agency, Olton, Solihull
The potential adverse effects on human health posed by exposure to bioaerosols emitted from composting facilities is a growing concern, particularly as more waste is being diverted from landfill [1]. At present, site monitoring is carried out using filtration or impaction samplers [2] but this only provides a snapshot of emissions in space and time. Dispersion models could play a valuable, complementary role to bioaerosol monitoring by providing a more continual overview of bioaerosol concentrations under different meteorological and operational scenarios. However, only modest progress has been made to date when applying a dispersion model in this context [3] and currently there is a lack of confidence in our ability to deploy atmospheric dispersion models to predict the dispersion of bioaerosols emitted from composting facilities.
Previous research has found that bioaerosol concentrations are underestimated when using the ADMS dispersion model [3]. It is suggested that this is mainly due to lack of detailed characterisation of the source term, and uncertainties regarding how to represent this type of scenario in the dispersion model. A detailed scenario-specific sensitivity analysis is necessary to identify the significant model input parameters to commence model optimization and validation. Alongside this, source data collection, which has also been recognised as a contributor to model underestimations, is needed to initiate modelling improvements.
1. Sykes, P., Jones, K., and Wildsmith, J. D., Resources, conservation and recycling, 52, 410, 2007.
2. Association for Organics Recycling: A standardised protocol for the monitoring of bioaerosols at open composting sites, Association for Organics Recycling, UK, 2009.
3. Drew, G. H., Tamer Vestlund., A. T., Jordinson, G., Taha, M. P. M., Smith, R., Tyrrel, S., Longhurst, P. J., and Pollard, S. J. T., In: Proceedings Sardinia 2007, Eleventh International waste management and landfill symposium. CISA, Environmental Sanitary Engineering Centre, Italy, 2007.
14. Characterization of Personal Exposures to and Indoor Concentrations of VOCs, PM2.5, PAHs, Quinones and Black Carbon
B. A. Macias-Hernandez, J. M. Delgado-Saborit and R. M. Harrison, School of Geography, Earth and Environmental Sciences, University of Birmingham.
There is growing public awareness regarding the risks associated with poor indoor air quality in the home and workplace.1,2 The aim of this study is to measure personal exposures in indoor environments and to estimate the lung doses of several pollutants of interest. Forty-five healthy, non-smoking adult subjects will be recruited, selected according to their likely different exposures to organic pollutants. The volunteers are grouped, for example, into (i) subjects occupationally exposed to benzene, (ii) subjects living or working in new buildings, and (iii) a control group. Volunteers are request to carry a briefcase containing sampling equipment for a period of 24 hours and to complete time-activity diaries.
Andrew Brown (1), Sanja Potgieter-Vermaak (1,2), Judith Barret (1) and Rene Van Grieken (3)
1 Division of Chemistry & Environmental Science, Manchester Metropolitan University.
2 School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa.
3 Department of Chemistry, University of Antwerp, Belgium.
In vitro and animal toxicological studies have confirmed that the chemical composition of inhaled particles play a major role in their toxic, genotoxic and carcinogenic mechanisms, but the component-specific toxic effects are still not understood. Particle-bound airborne transition metals can also lead to the production of Reactive Oxygen Species in lung tissue; a special concern amongst particularly susceptible cohorts (children and elderly). Thus the bioaccessibility of the fine fraction aerosol is evidently of importance for public health.
Size-fractioned (<38, 38–63, 63–125 μm) road dust samples collected from one of the highest trafficked roads in the United Kingdom (and believed to be one of the busiest bus routes in Europe) were characterised for their bulk elemental composition with EDXRF, ICP-OES and ICP-MS, and their molecular composition with micro Raman spectroscopy (MRS). It was found that the fine fraction (<38 μm) had the highest Pb (238 ppm) and Cr (171 ppm) concentrations. Concentrations of both Pb and Cr decreased substantially in the largest particle size fractions (from 279 ppm in particles with diameters Dp <38 μm to 13 ppm in particles with Dp <1mm for lead; and from 171 ppm for Dp <38 μm to 91 ppm for Dp <1mm for chromium). The MRS data showed that the Cr was mostly present as lead chromate and therefore in the Cr(VI) oxidation state. Apart from rather alarmingly high concentrations of oxidative stressors (Cu, Fe, Mn) obtained from the elemental analysis, the carcinogenic potential of the respirable fraction is evident from the MRS data. These same fractions underwent in vitro testing to assess the mobility of toxic and carcinogenic components by leaching with artificial body fluids. Leachates were analysed for Cr, Cu, Zn, V, Pb, Mn, Cd, Fe, Ni, Al and As concentrations at time intervals from 1 hour to 8 weeks. In general, most of the elements leached in the ppb range and concentrations decreased with increasing particle size. Although the mobility in the artificial body fluids of the various elements varied, up to 19% Cr, 47% Pb and 87% Ni were released.
13. Improving the Modeling of Bioaerosol Dispersion from Green Waste Composting Facilities
Philippa Douglas1, Gillian Drew1, Rob Kinnersley2, Kerry Walsh2, Phil Longhurst1 and Sean Tyrrel1
1.Centre for Energy and Resource Technology, School of Applied Sciences, Cranfield University
2.Environment Agency, Olton, Solihull
The potential adverse effects on human health posed by exposure to bioaerosols emitted from composting facilities is a growing concern, particularly as more waste is being diverted from landfill [1]. At present, site monitoring is carried out using filtration or impaction samplers [2] but this only provides a snapshot of emissions in space and time. Dispersion models could play a valuable, complementary role to bioaerosol monitoring by providing a more continual overview of bioaerosol concentrations under different meteorological and operational scenarios. However, only modest progress has been made to date when applying a dispersion model in this context [3] and currently there is a lack of confidence in our ability to deploy atmospheric dispersion models to predict the dispersion of bioaerosols emitted from composting facilities.
Previous research has found that bioaerosol concentrations are underestimated when using the ADMS dispersion model [3]. It is suggested that this is mainly due to lack of detailed characterisation of the source term, and uncertainties regarding how to represent this type of scenario in the dispersion model. A detailed scenario-specific sensitivity analysis is necessary to identify the significant model input parameters to commence model optimization and validation. Alongside this, source data collection, which has also been recognised as a contributor to model underestimations, is needed to initiate modelling improvements.
1. Sykes, P., Jones, K., and Wildsmith, J. D., Resources, conservation and recycling, 52, 410, 2007.
2. Association for Organics Recycling: A standardised protocol for the monitoring of bioaerosols at open composting sites, Association for Organics Recycling, UK, 2009.
3. Drew, G. H., Tamer Vestlund., A. T., Jordinson, G., Taha, M. P. M., Smith, R., Tyrrel, S., Longhurst, P. J., and Pollard, S. J. T., In: Proceedings Sardinia 2007, Eleventh International waste management and landfill symposium. CISA, Environmental Sanitary Engineering Centre, Italy, 2007.
14. Characterization of Personal Exposures to and Indoor Concentrations of VOCs, PM2.5, PAHs, Quinones and Black Carbon
B. A. Macias-Hernandez, J. M. Delgado-Saborit and R. M. Harrison, School of Geography, Earth and Environmental Sciences, University of Birmingham.
There is growing public awareness regarding the risks associated with poor indoor air quality in the home and workplace.1,2 The aim of this study is to measure personal exposures in indoor environments and to estimate the lung doses of several pollutants of interest. Forty-five healthy, non-smoking adult subjects will be recruited, selected according to their likely different exposures to organic pollutants. The volunteers are grouped, for example, into (i) subjects occupationally exposed to benzene, (ii) subjects living or working in new buildings, and (iii) a control group. Volunteers are request to carry a briefcase containing sampling equipment for a period of 24 hours and to complete time-activity diaries.
A total of 46 filters were sampled from the first group of 16 volunteers recruited during winter 2011. Marginally higher concentrations of PM2.5 (particulate matter with diameters ≤ 2.5 μm) were found in the workplace, with an arithmetic mean of 30 ± 15 µg/m3. These values are generally higher than the standard proposed by the World Health Organization (25 µg/m3) [3]. Results from a total of 4,455 observations (sampling time of 5 minutes each) showed that the highest concentrations of black carbon were found outdoors (5,163 ± 3,121 ng/m3), whilst the highest concentrations measured indoors were in pubs and restaurants (2,901 ± 4,229 ng/m3).
1. Ward, T., Underberg, H. et al., Environmental Monitoring and Assessment, 153, 119, 2009.
2. Bernstein, J. A., Alexis, N. et al., Journal of Allergy and Clinical Immunology, 121, 585, 2008.
3. Environmental Protection Agency, An Office Building Occupant's Guide to Indoor Air Quality, Washington DC, 1997.
15. Assessment of Vehicular Profiles vis-à-vis Real-world Traffic Emissions
Pallavi Pant and Roy M. Harrison, School of Geography, Earth and Environmental Studies, University of Birmingham
Receptor modelling, particularly the chemical mass balance (CMB) model is one of the often-used tools for estimation of source contributions to concentrations of particulate matter in ambient air. Further, the CMB model relies to a large extent on the accuracy of the source profiles used as an input. Most gasoline and diesel engine source profiles are generated through emission characterizations performed under laboratory conditions. However, significant differences have been observed between laboratory-testing and real-world mixed source traffic emissions [1,2].
To achieve the twin goals of assessing existing source profiles with respect to the ambient traffic emissions data and preparation of a mixed-source traffic profile for London, samples were collected at two different urban sites (background and roadside) in London. It was assumed that if all other sources contribute to the same extent at both sites, the increment in marker concentrations would be due to traffic emissions. Ambient organic marker data from London was compared with published source profiles using ratio-ratio plots. Results indicate that mixed-traffic profiles generated using data collected from the ambient environment show a greater similarity with ambient concentrations. This can be attributed to the relative similarity to the real-world driving and emission conditions. Also, while the lab-generated source profiles vary significantly from each other in some cases, most of the real-world profiles show high levels of similarity. A local traffic emissions profile has also been prepared for London which is being used for further data analysis.
1. Ancelet, T., Davy, P. K., Trompetter, W. J., Markwitz, A., and Weatherburn, D. C., Atmospheric Environment, 45, 4463, 2011.
2. Yan, B., Zheng, M., Hu, Y. et al., Environmental Science &Technology, 43, 4287, 2009.
2. Bernstein, J. A., Alexis, N. et al., Journal of Allergy and Clinical Immunology, 121, 585, 2008.
3. Environmental Protection Agency, An Office Building Occupant's Guide to Indoor Air Quality, Washington DC, 1997.
15. Assessment of Vehicular Profiles vis-à-vis Real-world Traffic Emissions
Pallavi Pant and Roy M. Harrison, School of Geography, Earth and Environmental Studies, University of Birmingham
Receptor modelling, particularly the chemical mass balance (CMB) model is one of the often-used tools for estimation of source contributions to concentrations of particulate matter in ambient air. Further, the CMB model relies to a large extent on the accuracy of the source profiles used as an input. Most gasoline and diesel engine source profiles are generated through emission characterizations performed under laboratory conditions. However, significant differences have been observed between laboratory-testing and real-world mixed source traffic emissions [1,2].
To achieve the twin goals of assessing existing source profiles with respect to the ambient traffic emissions data and preparation of a mixed-source traffic profile for London, samples were collected at two different urban sites (background and roadside) in London. It was assumed that if all other sources contribute to the same extent at both sites, the increment in marker concentrations would be due to traffic emissions. Ambient organic marker data from London was compared with published source profiles using ratio-ratio plots. Results indicate that mixed-traffic profiles generated using data collected from the ambient environment show a greater similarity with ambient concentrations. This can be attributed to the relative similarity to the real-world driving and emission conditions. Also, while the lab-generated source profiles vary significantly from each other in some cases, most of the real-world profiles show high levels of similarity. A local traffic emissions profile has also been prepared for London which is being used for further data analysis.
1. Ancelet, T., Davy, P. K., Trompetter, W. J., Markwitz, A., and Weatherburn, D. C., Atmospheric Environment, 45, 4463, 2011.
2. Yan, B., Zheng, M., Hu, Y. et al., Environmental Science &Technology, 43, 4287, 2009.
Section 4: Environmental electrochemistry
16. Investigation of the Uranium Redox Couple by Voltammetric Techniques
Mei Qi CHEW, Nick D. M. Evans and Roger J. Mortimer, Department of Chemistry, University of Loughborough
Uranium is a redox-sensitive element and its chemical properties depend considerably on pH and oxidation state. This work is concerned with the oxidation-reduction behaviour of uranium in a potential radioactive Geological Disposal Facility (GDF). It is important to understand the redox behaviour of uranium in order to evaluate its mobility in the GDF environment and the consequent security of its disposal. The low solubility of uranium at high pH makes conventional experimental voltammetric techniques difficult to use. The main aim of this work is therefore to investigate the chemical effects (pH, addition of chelating agents etc) on the behaviour of the uranium couple and optimise the cyclic voltammetric technique [1,2].
Mei Qi CHEW, Nick D. M. Evans and Roger J. Mortimer, Department of Chemistry, University of Loughborough
Uranium is a redox-sensitive element and its chemical properties depend considerably on pH and oxidation state. This work is concerned with the oxidation-reduction behaviour of uranium in a potential radioactive Geological Disposal Facility (GDF). It is important to understand the redox behaviour of uranium in order to evaluate its mobility in the GDF environment and the consequent security of its disposal. The low solubility of uranium at high pH makes conventional experimental voltammetric techniques difficult to use. The main aim of this work is therefore to investigate the chemical effects (pH, addition of chelating agents etc) on the behaviour of the uranium couple and optimise the cyclic voltammetric technique [1,2].
This work describes the effect of changing pH on the redox behaviour and the reversibility of the various uranium redox couples using voltammetric techniques and optimisation of the method. Cyclic voltammetry did not produce satisfactory results at low concentrations of uranium (~10–5 mol dm–3). The concentrations of uranium were then increased to the mM range and subsequently more defined and reproducible voltammetric waves were obtained.
Experiments were performed using uranyl nitrate across a pH range from 1 to 14 in the presence and absence of sodium carbonate.2 The kinetic effects, mechanisms and thermodynamics of the redox reactions were investigated. The effects of the addition of ethylenediaminetetraacetic acid (EDTA) were subsequently studied, and the electrode potentials of the redox couple in non-ligand and ligand systems were compared. The effects of organic complex formation on the redox behaviour of the uranium couple were also observed.
1. Capdevila, H. and Vitorge, P., J. Radioanal. Nucl. Chem., 143, 403, 1990.
2. Clark, D. L., Hobart, D.E. and Neu, M. P., Chem. Rev., 95, 25,1995.
ABSTRACTS WERE PROVIDED BY THE FORUM DELEGATES. EDITING BY STEPHEN BALL and WILLIAM BLOSS
1. Capdevila, H. and Vitorge, P., J. Radioanal. Nucl. Chem., 143, 403, 1990.
2. Clark, D. L., Hobart, D.E. and Neu, M. P., Chem. Rev., 95, 25,1995.
ABSTRACTS WERE PROVIDED BY THE FORUM DELEGATES. EDITING BY STEPHEN BALL and WILLIAM BLOSS