Transforming CO2 into construction products
Colin D. Hills and Nimisha Tripathi
University of Greenwich
[email protected], [email protected]
ECG Bulletin July 2023
University of Greenwich
[email protected], [email protected]
ECG Bulletin July 2023
The UK government’s recent decision to
invest £20 billion in a carbon capture,
utilisation, and storage (CCUS) infrastructure
fund, is a significant boost for using
anthropogenic CO2 gas to manufacture
consumer products as diverse as jet fuel or
building materials. The UK has been
leading in the latter area since 2012, when
the world’s first full-scale facility for
manufacturing carbonated construction
aggregates was established in East Anglia.
Neither the potential to transform
gigatonnes of anthropogenic CO2 into
limestone, nor the similarities between
mineralisation technology and natural
Earth processes are fully appreciated.
The use of man-made cementitious mortars extends back 10,000 years (1). Around 25 BC, Vitruvius, a Roman architect and engineer, was recommending combinations of volcanic ash and lime mortars for construction projects; some are still in service today (2,3). In part, the longevity of these Roman mortars is the result of the reaction of carbon dioxide gas in the atmosphere with quicklime to form
calcium carbonate (Eq.1, below). The resulting carbonated mortar is more thermodynamically stable, durable, and relatively forgiving of structural movement.
calcium carbonate (Eq.1, below). The resulting carbonated mortar is more thermodynamically stable, durable, and relatively forgiving of structural movement.
CaO + CO2 ⇌ CaCO3 Eq.1
What is not fully recognised are the parallels between
manmade carbonated materials and those made by natural
processes. In nature, CO2 is mineralised in the weathering
of rock, or by chemical and biologically-mitigated reactions
in the oceans or in soil.
These reactions result in carbonate being effectively stored in the geosphere over geological time scales - harmful gas is permanently ‘locked up’ and thus, cannot adversely impact the climate and ecosystems.
Our planet is good at partitioning carbon and the billions of
Gts of carbon stored in the geosphere represent about 99%
of the Earth’s total reserves. According to the Deep Carbon
Observatory, these carbon reserves are in the order of 1.85
x 1018 tonnes(4), with most being carbonates in sedimentary
rocks – of which limestone accounts for about 4.0 x 10
tonnes(5). The feedback time for the amelioration of
anthropogenic CO2 is in the order
of hundreds of millennia, thus,
for us to moderate our climate,
industrialisation of carbon
transformation is required.
CCUS has potential to make a
difference in the shorter-term
by processing anthropogenic
carbon into manufactured
products. As carbon utilisation
is complementary to carbon storage, both strategies are
needed to meet the UK’s emission reduction goals, reflected
in recent investment by the UK Government(6,7).
Managed carbonation
Chemical processes that can transform CO2 can involve the use of high temperatures and pressures, and chemical reagents. To keep associated emissions to a minimum, energy intensive processes utilising fossil fuels should be avoided wherever possible. Our work is focused on using ambient temperatures and pressures during processing (9). The resultant calcium carbonate-based products have high positive environmental carbon offsets via the direct removal and permanent capture of flue-gas derived CO2. Indirect offsets include reduced use of virgin materials and the avoidance of quarrying, crushing, and transportation. Many industrial alkaline waste streams are excellent substrates to mineralise CO2 upon allowing wastes to also be risk managed and diverted from landfill. The estimates of suitable waste streams worldwide for use as mineralisation vary from 1-4 Gt/yr. These process wastes tend to be closely associated with point-source CO2 emissions, creating an opportunity to combine solid and gaseous emissions at one site. In Europe, there is potential to sequester ca. 7.8 Mt/yr of CO2 in 79 Mt of alkaline residues from five different waste streams: municipal solid waste incinerator; biomass; paper and oil shale ashes; steel slag; and construction and demolition waste. Waste arisings such as these are carefully documented (10).
Chemical processes that can transform CO2 can involve the use of high temperatures and pressures, and chemical reagents. To keep associated emissions to a minimum, energy intensive processes utilising fossil fuels should be avoided wherever possible. Our work is focused on using ambient temperatures and pressures during processing (9). The resultant calcium carbonate-based products have high positive environmental carbon offsets via the direct removal and permanent capture of flue-gas derived CO2. Indirect offsets include reduced use of virgin materials and the avoidance of quarrying, crushing, and transportation. Many industrial alkaline waste streams are excellent substrates to mineralise CO2 upon allowing wastes to also be risk managed and diverted from landfill. The estimates of suitable waste streams worldwide for use as mineralisation vary from 1-4 Gt/yr. These process wastes tend to be closely associated with point-source CO2 emissions, creating an opportunity to combine solid and gaseous emissions at one site. In Europe, there is potential to sequester ca. 7.8 Mt/yr of CO2 in 79 Mt of alkaline residues from five different waste streams: municipal solid waste incinerator; biomass; paper and oil shale ashes; steel slag; and construction and demolition waste. Waste arisings such as these are carefully documented (10).
Kinetic constraints upon carbonation have been summarised by the IPCC (11) who concluded that high-grade energy is required to drive the mineral carbonation process and that (without refining), direct carbonation with flue gas must include raised pressures. This is not strictly true as the direct use of flue gas is achieved under ambient conditions (12,13).
Managed carbonation pathways
It is useful to describe managed carbonation as falling into three applications based on the amount of water use in the reaction process:
(i) Wet carbonation involves a water/solids ratio above 0.4w/w (total wt.). This route is normally applied to mineral suspensions in aqueous solution in which CO2 is dissolved in a batch reactor under mild conditions, but can involve elevated temperatures and pressures. The products tend to be relatively pure, fine-grained precipitated calcium carbonate (14).
(ii) Semi-dry carbonation (or direct carbonation) using water/solid ratios below < 0.4 w/w, typically < 0.3 w/w (total weight). This reaction environment enables the formation of a thin film of moisture on the surface of minerals to facilitate rapid dissolution and ionisation of CO2, enabling carbonation to proceed quickly. Monolithic products may be manufactured.
(iii) Assisted carbonation, where temperatures and pressures are significantly raised above ambient conditions, possibly exceeding 250°C and 2 MPa. Processing may involve digestion, use of proprietary solvents/ chemicals (which, in themselves may require energy intensive forms of preparation/synthesis), or the application of supercritical CO2 (15). Diverse products may be manufactured.
It is useful to describe managed carbonation as falling into three applications based on the amount of water use in the reaction process:
(i) Wet carbonation involves a water/solids ratio above 0.4w/w (total wt.). This route is normally applied to mineral suspensions in aqueous solution in which CO2 is dissolved in a batch reactor under mild conditions, but can involve elevated temperatures and pressures. The products tend to be relatively pure, fine-grained precipitated calcium carbonate (14).
(ii) Semi-dry carbonation (or direct carbonation) using water/solid ratios below < 0.4 w/w, typically < 0.3 w/w (total weight). This reaction environment enables the formation of a thin film of moisture on the surface of minerals to facilitate rapid dissolution and ionisation of CO2, enabling carbonation to proceed quickly. Monolithic products may be manufactured.
(iii) Assisted carbonation, where temperatures and pressures are significantly raised above ambient conditions, possibly exceeding 250°C and 2 MPa. Processing may involve digestion, use of proprietary solvents/ chemicals (which, in themselves may require energy intensive forms of preparation/synthesis), or the application of supercritical CO2 (15). Diverse products may be manufactured.
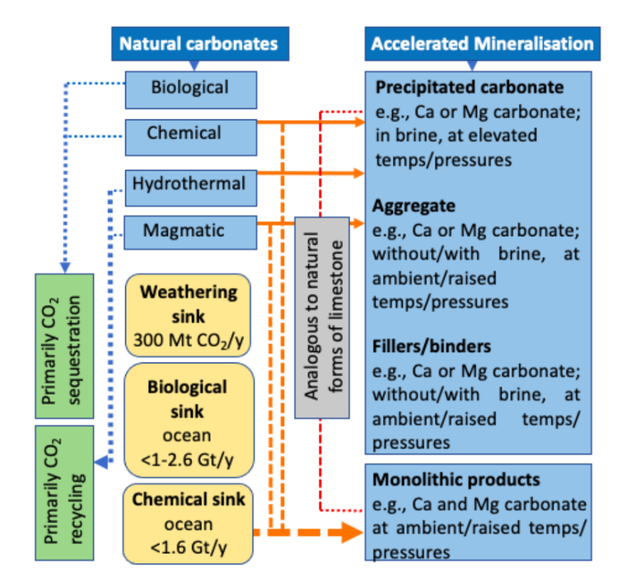
Figure 1 summarises the relationship between natural and managed carbonation, and potential net gain via CO2 sequestration (direct and indicative indirect offsets (16,17,18)).
Our interest is the semi-dry carbonation of alkaline solid indus trial process residues, con taining mineral components that readily react with CO2 gas. As outlined in (ii) above, the products are analogous to natural carbonates formed at or near the Earth’s surface, where temperatures and pressures are generally low, and the prevalent chemical environment tends to be alkaline/saline. The products of managed mineralisation include precipitated carbonates, light-weight aggregates, fillers/binders, or shaped monoliths.
|
Notably, the transmitted light micrograph shown in Figure
2 is of a laboratory-manufactured carbonated aggregate comprising an air pollution control residue. The concentric layered structure of the aggregate is built around a central core and is analogous in structure to that displayed by pisoliths formed in terrestrial, lacustrine, and marine environments (19,20). The natural formation of limestone over geological time scales and the production of carbonated products via mineralisation are demonstrably analogous, but the latter is achieved in minutes, whereas the former are made over extended timescales by natural processes including chemical, biological, and magmatic. |
Conclusion
Directly combining solid and gaseous wastes from single or multiple emitters is circular. Technology established in the UK can be extended to different industrial solid waste feedstock, and result in manufactured carbonated construction products for a variety of applications. As indicated by GCI (21), there is potential to utilise 3-6 Gt of anthropogenic CO2 in construction materials and generate annual revenues of $1 trillion each year.
Directly combining solid and gaseous wastes from single or multiple emitters is circular. Technology established in the UK can be extended to different industrial solid waste feedstock, and result in manufactured carbonated construction products for a variety of applications. As indicated by GCI (21), there is potential to utilise 3-6 Gt of anthropogenic CO2 in construction materials and generate annual revenues of $1 trillion each year.
References
1. D. Carran, J. Hughes, A. Leslie, C. Kennedy, A short history of the use of lime as a building material beyond Europe and North America. International Journal of Architectural Heritage, 2012 6(2), 117–146. https://doi.org/10.1080/15583058.2010.511694
2. Limebase. http://www.limebase.co.uk/guides/the-history-of-lime-mortar/#:~:text=The%20Romans%20developed%20a%20hydraulic,to%20pozzolan%20for%20underwater%20works [Accessed April 2023].
3. L. M. Seymour, J. Maragh, P. Sabatini, M. Di Tommaso, J. C. Weaver, A. Masic, Hot mixing: Mechanistic insights into the durability of ancient Roman concrete. Science Advances, 2023 9 (1), eadd1602. https://doi.org/10.1126/sciadv.add1602
4. Deep Carbon Observatory. Deep carbon observatory: A decade of discovery. Deep Carbon Observatory Secretariat . 2019 https://doi.org/10.17863/CAM.44064
5. L. R. Kump, J. F. Kasting, & R. G. Crane, The Earth System, Pearson pubs, London, 2004
6. The ten point plan for a green Industrial Revolution, http://www.gov.uk/government/publications/the-ten-point-plan-for-a-green-industrial-revolution/title [Accessed December 2020]
7. Upstreamonline, http://www.upstreamonline.com/energy-transition/uk-earmarks-20-billion-for-carbon-capture-and-storage-funding/2-1-1420257 [Accessed: April 2023]
8. United Nations. Sand and sustainability: 10 strategic recommendations to avert a crisis. Global resource information database. EP. 2022. https://unepgrid.ch/storage/app/media/Publications/2022sandandsustainabilityreportfinal.pdf
9. P. J. Gunning, C. D. Hills, and P. J. Carey, Production of lightweight aggregate from industrial waste and carbon dioxide. Waste Management, 2009, 29 (10), 2722–2728. https://doi.org/10.1016/j.wasman.2009.05.021
10. Eurostat, https://ec.europa.eu/eurostat/en/web/products-key-figures/w/ks-ei-23-001 [Accessed July 2023]
11. IPCC. IPCC special report on carbon dioxide capture and
storage. Prepared by working group B. Metz, O. Davidson, H. C. de Coninck, M. Loos & L. A. Meyer (Eds.), III of the Intergovernmental Panel on Climate Change, 2005, p. 442. Cambridge University Press.
12. J. H. Kim, and W. T. Kwon, Semi-dry carbonation process using fly ash from solid refused fuel power Plant. Sustainability, 2019, 11 (3), 908. https://doi.org/10.3390/su11030908
13. Carbon, 8, http://www.carbon8.co.uk [Accessed April 2023]
1. D. Carran, J. Hughes, A. Leslie, C. Kennedy, A short history of the use of lime as a building material beyond Europe and North America. International Journal of Architectural Heritage, 2012 6(2), 117–146. https://doi.org/10.1080/15583058.2010.511694
2. Limebase. http://www.limebase.co.uk/guides/the-history-of-lime-mortar/#:~:text=The%20Romans%20developed%20a%20hydraulic,to%20pozzolan%20for%20underwater%20works [Accessed April 2023].
3. L. M. Seymour, J. Maragh, P. Sabatini, M. Di Tommaso, J. C. Weaver, A. Masic, Hot mixing: Mechanistic insights into the durability of ancient Roman concrete. Science Advances, 2023 9 (1), eadd1602. https://doi.org/10.1126/sciadv.add1602
4. Deep Carbon Observatory. Deep carbon observatory: A decade of discovery. Deep Carbon Observatory Secretariat . 2019 https://doi.org/10.17863/CAM.44064
5. L. R. Kump, J. F. Kasting, & R. G. Crane, The Earth System, Pearson pubs, London, 2004
6. The ten point plan for a green Industrial Revolution, http://www.gov.uk/government/publications/the-ten-point-plan-for-a-green-industrial-revolution/title [Accessed December 2020]
7. Upstreamonline, http://www.upstreamonline.com/energy-transition/uk-earmarks-20-billion-for-carbon-capture-and-storage-funding/2-1-1420257 [Accessed: April 2023]
8. United Nations. Sand and sustainability: 10 strategic recommendations to avert a crisis. Global resource information database. EP. 2022. https://unepgrid.ch/storage/app/media/Publications/2022sandandsustainabilityreportfinal.pdf
9. P. J. Gunning, C. D. Hills, and P. J. Carey, Production of lightweight aggregate from industrial waste and carbon dioxide. Waste Management, 2009, 29 (10), 2722–2728. https://doi.org/10.1016/j.wasman.2009.05.021
10. Eurostat, https://ec.europa.eu/eurostat/en/web/products-key-figures/w/ks-ei-23-001 [Accessed July 2023]
11. IPCC. IPCC special report on carbon dioxide capture and
storage. Prepared by working group B. Metz, O. Davidson, H. C. de Coninck, M. Loos & L. A. Meyer (Eds.), III of the Intergovernmental Panel on Climate Change, 2005, p. 442. Cambridge University Press.
12. J. H. Kim, and W. T. Kwon, Semi-dry carbonation process using fly ash from solid refused fuel power Plant. Sustainability, 2019, 11 (3), 908. https://doi.org/10.3390/su11030908
13. Carbon, 8, http://www.carbon8.co.uk [Accessed April 2023]
14. H. Ostovari, A. Sternberg, and A. Bardow, Rock ‘n’ use of
CO2: carbon footprint of carbon capture and utilization
by mineralization Sustainable Energy and Fuels, 2020,
4 ( 9 ), 4482–4496. https://doi.org/10.1039/D0SE00190B
15. S. Chakraborty, and B. W. Jo, B. W. In Carbon Dioxide Sequestration in Cementitious Construction Materials, ed. F. Pacheco-Torgal, Caijun Shi & A. Palomo Sanchez, Woodhead Publishing, Cambridge, 2018, pp. 39-64 https://doi.org/10.1016/B978-0-08-102444-7.00003-4
16. S. A. Rackley, Carbon Capture and Storage (2nd ed), Butterworth-Heinemann, Oxford, 2017. https://doi.org/10.1016/C2009019306-6
17. L. Ji, and H. Yuin Carbon Dioxide Sequestration in Cementitious Construction Materials, ed. F. Pacheco-Torgal, Caijun Shi & A. Palomo Sanchez, Woodhead Publishing, Cambridge, 2018, pp. 13–37. https://doi.org/10.1016/B978-0-08-102444-7.00002-2
16. S. A. Rackley, Carbon Capture and Storage (2nd ed), Butterworth-Heinemann, Oxford, 2017. https://doi.org/10.1016/C2009019306-6
17. L. Ji, and H. Yuin Carbon Dioxide Sequestration in Cementitious Construction Materials, ed. F. Pacheco-Torgal, Caijun Shi & A. Palomo Sanchez, Woodhead Publishing, Cambridge, 2018, pp. 13–37. https://doi.org/10.1016/B978-0-08-102444-7.00002-2
18. C. D. Hills, N. Tripathi, and P. J. Carey, Managed pathways
for CO2 mineralisation: analogy with nature and
potential contribution to CCUS-led reduction targets.
Faraday Discussions, 2021, 230, 152–171. https://doi.org/10.1039/d0fd00142b
19. D. F. Lascelles, The origin of terrestrial pisoliths and pisolitic iron ore deposits: Raindrops and sheetwash in a semi-arid environment. Sedimentary Geology, 2021, 341, 232–244. https://doi.org/10.1016/j.sedgeo.2016.04.015 0037-0738.
20. D. K. Richter, In Coated Grains, ed. T. M. Peryt, Springer, New York, 1983. https://doi.org/10.1007/978-3-642-68869-0_7
20. D. K. Richter, In Coated Grains, ed. T. M. Peryt, Springer, New York, 1983. https://doi.org/10.1007/978-3-642-68869-0_7
21. GCI, Global CO2 Initiative, http://www.globalco2initiative.org [Retrieved April 2023].