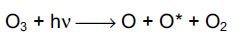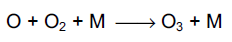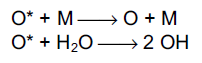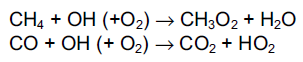Climate change, methane and ozone
Introduction
Methane (CH4) and tropospheric ozone (O3) are the two most important trace gases involved in global warming, after carbon dioxide (CO2). The concentrations of both of these gases have risen substantially during the industrial era, owing to the extraction and combustion of fossil fuels (IPCC, 2001). Their levels in the troposphere are closely linked via chemical reactions involving water vapour, and changes in the concentration of one will affect the concentrations of the other (Johnson et al., 2001, 2002). Oxidation of methane is responsible for the majority of the ozone formation in the troposphere (West and Fiore, 2005). Production of the hydroxyl radical (OH), which is responsible for almost all the oxidation of methane in the troposphere, is controlled by the levels of ozone. In this article, the feedbacks between methane and ozone will be explained, and the impacts of climate change via water vapour levels will be discussed.
Methane (CH4) and tropospheric ozone (O3) are the two most important trace gases involved in global warming, after carbon dioxide (CO2). The concentrations of both of these gases have risen substantially during the industrial era, owing to the extraction and combustion of fossil fuels (IPCC, 2001). Their levels in the troposphere are closely linked via chemical reactions involving water vapour, and changes in the concentration of one will affect the concentrations of the other (Johnson et al., 2001, 2002). Oxidation of methane is responsible for the majority of the ozone formation in the troposphere (West and Fiore, 2005). Production of the hydroxyl radical (OH), which is responsible for almost all the oxidation of methane in the troposphere, is controlled by the levels of ozone. In this article, the feedbacks between methane and ozone will be explained, and the impacts of climate change via water vapour levels will be discussed.
Ozone photochemistry
Ozone is photolysed by light at wavelengths smaller than 370 nm to produce an oxygen molecule and an oxygen atom. The latter may be produced in two forms, a ground state (O) or an energetically excited state (O*). At wavelengths less than 310 nm, most of the oxygen atoms are in the excited state (Wayne, 1985).
Ozone is photolysed by light at wavelengths smaller than 370 nm to produce an oxygen molecule and an oxygen atom. The latter may be produced in two forms, a ground state (O) or an energetically excited state (O*). At wavelengths less than 310 nm, most of the oxygen atoms are in the excited state (Wayne, 1985).
The ground-state oxygen atom will react with oxygen molecules to regenerate ozone,
where M represents a third body (typically an oxygen or nitrogen molecule). The excited oxygen atom may either be quenched back to the ground state via collisions with a third body, or it may react with water vapour to produce two hydroxyl radicals,
Hydroxyl radicals are of fundamental importance in the chemistry of the troposphere. They react with almost all emitted pollutants, and such a reaction is the first step in the oxidation of pollutants. Taking the simplest hydrocarbon, methane, oxidation produces the intermediate species formaldehyde (HCHO) and then carbon monoxide. Carbon monoxide is oxidised exclusively by hydroxyl radicals to form carbon dioxide. The initial step for methane is shown below, together with the reaction between hydroxyl radicals and carbon monoxide:
The methylperoxy radical (CH3O2) reacts further to produce formaldehyde, HCHO. The subsequent reactions of formaldehyde and hydroperoxy radicals (HO2) will reform the hydroxyl radical. The oxidation of methane and carbon monoxide is therefore catalytic, as no net destruction of hydroxyl radicals occurs (Wayne, 1985).
Impacts of climate change on methane
The concentration of water vapour in the troposphere is controlled by a number of complex processes. In the lowest part of the troposphere, called the boundary layer, the mass of water vapour tends to increase with increasing temperature. Outside of the boundary layer, water vapour levels are controlled by many different physical processes. An increase in temperature may not lead to more water vapour in this region. However, overall, climate models tend to predict greater water vapour levels in the troposphere with increasing temperature.
The concentration of water vapour in the troposphere is controlled by a number of complex processes. In the lowest part of the troposphere, called the boundary layer, the mass of water vapour tends to increase with increasing temperature. Outside of the boundary layer, water vapour levels are controlled by many different physical processes. An increase in temperature may not lead to more water vapour in this region. However, overall, climate models tend to predict greater water vapour levels in the troposphere with increasing temperature.
|
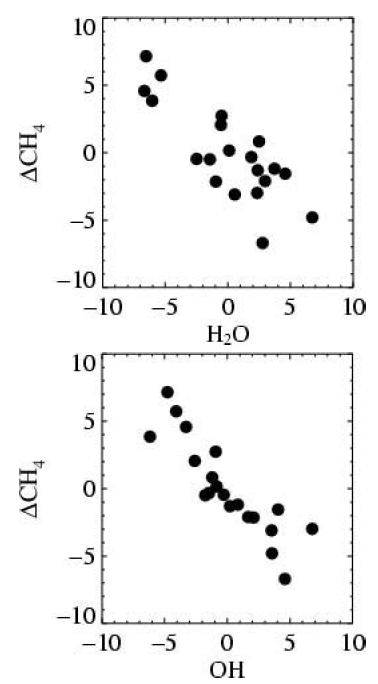
If water vapour levels rise, the production of hydroxyl radicals from the excited state oxygen atom (O*) is likely to increase. The oxidation rates of methane and carbon monoxide will also proceed more quickly, owing to the higher production rate of hydroxyl radicals. To investigate this feedback, a chemistry model was coupled to the Hadley Centre’s climate model HadCM3. Levels of carbon dioxide were increased according to the IPCC SRES A2 emissions scenario (IPCC, 2000). This coupled chemistry-climate model was integrated forward in time. Scatter plots of the change in global mean methane levels against total atmospheric water vapour and global mean hydroxyl radical levels are shown in Figure 1 for the first 20 years of the simulation. In both cases, a significant correlation exists between the change in methane levels, and each of the water vapour amount and hydroxyl radical concentrations. A similar plot using global mean surface temperature (not shown) showed a distinctly poorer correlation. These results show that the level of water vapour in the troposphere exerts an important influence on the methane lifetime, via the production of the hydroxyl radical. As the climate continues to warm, this loss rate is likely to increase further. Overall, climate change results in greater production of hydroxyl radicals, and reduces levels of methane via increases in water vapour. However, this reduction is not enough to offset increased emissions from anthropogenic activities. By 2030, the reduction in methane levels owing to climate change effects is only 1 – 2%. For 2050, the methane levels are only 4% smaller when climate change effects are included (Johnson et al., 2001).
Future ozone levels Ozone levels in the troposphere have risen substantially since pre-industrial times (Volz and Kley, 1988), and have continued to rise in more recent times (Vingarzan, 2004). This rise in ozone levels is caused by increased emissions of precursor gases, such as nitrogen oxides (NOx), carbon monoxide and hydrocarbons (Dentener et al., 2005, 2006). |
In the future, as the climate continues to warm, the chemical reactions involved in producing ozone will proceed more quickly. If emissions of precursors also increase, ozone levels in the future will be even larger. However, owing to the increased water vapour in a future climate, surface ozone levels at least will be partly reduced via the reactions of the excited oxygen atom with water vapour, as discussed above (Johnson et al., 2001; Stevenson et al., 2006).
The competing effects of climate change and emissions increases on projected surface ozone levels for the year 2030 have been studied using a coupled chemistry-climate model. Three simulations were used: First, a control simulation (1) which used climate data and emissions suitable for the year 2000. The second simulation (2) used projected emissions for the year 2030, but the climate data for the year 2000. The third simulation (3) used projected emissions and a climate for the year 2030. The difference between the ozone levels from the second and third simulations will illustrate the impact of climate change alone on projected surface ozone levels. The emissions were taken from the IIASA Current Legislation scenario (Dentener et al., 2005). This scenario assumes the majority of countries will have implemented regulations requiring reductions in emissions of pollutants by 2030. These results are shown in Figure 2.
The top panel of Figure 2 shows projected changes in annual mean surface ozone levels owing to increased emissions of pollutants only (Simulation 2 – Simulation 1). Ozone levels have only risen slightly over much of Europe and North America, by between 1 and 2 ppb, owing to the assumed reductions in emission in the Current Legislation scenario. The rise in ozone levels is slightly greater over Africa, by 2 – 3 ppb. The largest increases under the scenario occur over India and south-east Asia, by up to 15 ppb. This large increase in ozone levels over this part of Asia is due to projected increases in emissions of primary pollutants from power generation and vehicle numbers.
The same change in surface ozone levels, but also including the effects of climate change, are shown in the middle panel of Figure 2 (Simulation 3 – Simulation 1). The patterns of change in the ozone levels are broadly similar to the top panel, with the largest increases once more over India and south-east Asia, and smaller increases over the continents in the northern hemisphere. However, the absolute increases in the ozone levels are smaller, and in some parts of the northern hemisphere ozone levels have decreased slightly, by 1 – 2 ppb. The inclusion of climate change has therefore decreased the projected surface ozone levels for 2030. The effect of climate change alone is shown in the lower panel of Figure 2. This panel is the difference between the upper and middle panels. Surface ozone levels have fallen by 1 – 2 ppb over most of the globe, although a rise of 1 – 2 ppb is predicted over parts of the Pacific Ocean. These results show that, as indicated above, climate change has generally decreased projected global surface ozone levels for 2030.
Summary
The levels of methane and ozone in the troposphere are closely linked via chemical reactions involving water vapour. Experiments using a coupled chemistry-climate model have shown that global methane levels are correlated with global water vapour levels, and global OH levels. Larger water vapour levels result in increased production of OH levels, and consequently smaller methane levels.
Further simulations using the coupled chemistry-climate model were used to study the effect of climate change on projected surface ozone levels for the year 2030. The water vapour levels are larger in the 2030 climate than in the 2000 climate. Climate change has reduced the projected ozone levels by 1 – 2 ppb over most of the globe. This reduction is due to the reaction between water vapour and excited state oxygen atoms. Owing to the increased water vapour levels, more excited state oxygen atoms react with water vapour, and fewer are quenched to the ground state to reform ozone. Hence, more hydroxyl radicals (OH) are made at the expense of ozone molecules, and ozone levels fall.
References
Dentener, F., Stevenson, D., Cofala, J., Mechler, R., Amann, M., Bergamaschi, P., Raes, F., and Derwent, R. 2005. The impact of air pollutant and methane emission controls on tropospheric ozone and radiative forcing: CTM calculations for the period 1990 – 2030. Atmos. Chem. Phys., 5, 1731-1755.
Dentener, F., Stevenson, D., Ellingsen, K., van Noije, T., Schultz, M., and co-authors. 2006. The global atmospheric environment for the next generation. Environ. Sci. Technol., 40, 3586-3594.
IPCC. 2000. Special Report on Emissions Scenarios: A special report of working group III of the intergovernmental panel on climate change (eds. N. Nakićenović et al.), Cambridge University Press, New York.
IPCC. 2001. Climate Change 2001: The Scientific Basis. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change (eds. J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, K. Maskell, and C. A. Johnson), Cambridge University Press, New York.
Johnson, C.E., Stevenson, D.S, Collins, W.J. and Derwent, R.G. 2001. Role of climate feedback on methane and ozone studied with a coupled Ocean-Atmosphere-Chemistry model. Geophys. Res. Lett., 28, 1723-1726.
Johnson, C.E., Stevenson, D.S., Collins, W.J., and Derwent, R.G. 2002. Interannual variability in methane growth rate simulated with a coupled ocean-atmosphere-chemistry model. Geophys. Res. Lett., 29, 1903, doi:10.1029/2002GL015269, 2002.
Stevenson, D.S., Dentener, F.J., Schultz, M.G., Ellingsen, K., Van Noije,
T.P.C., and co-authors. 2006. Multi-model ensemble simulations of present-day and near-future tropospheric ozone. J. Geophys. Res., 111, D084301, doi:10.1029/ 2005JD00638.
Vingarzan, R. 2004. A review of surface ozone background levels and trends.
Atmos. Environ., 38, 3431-3442.
Volz, A. and Kley, D. 1988. Evaluation of the Montsouris series of ozone measurements made in the nineteenth century. Nature, 332, 240-242.
Wayne, R. P. 1985. Chemistry of Atmospheres, Oxford University Press, Oxford, U.K.
West, J.J., and Fiore, A.M. 2005. Management of tropospheric ozone by reducing methane emissions. Environ. Sci. Technol., 39, 4685 -4691.
MICHAEL SANDERSON
Hadley Centre, Met Office, Fitzroy
Road, Exeter EX1 3PB
E-mail: [email protected]
The same change in surface ozone levels, but also including the effects of climate change, are shown in the middle panel of Figure 2 (Simulation 3 – Simulation 1). The patterns of change in the ozone levels are broadly similar to the top panel, with the largest increases once more over India and south-east Asia, and smaller increases over the continents in the northern hemisphere. However, the absolute increases in the ozone levels are smaller, and in some parts of the northern hemisphere ozone levels have decreased slightly, by 1 – 2 ppb. The inclusion of climate change has therefore decreased the projected surface ozone levels for 2030. The effect of climate change alone is shown in the lower panel of Figure 2. This panel is the difference between the upper and middle panels. Surface ozone levels have fallen by 1 – 2 ppb over most of the globe, although a rise of 1 – 2 ppb is predicted over parts of the Pacific Ocean. These results show that, as indicated above, climate change has generally decreased projected global surface ozone levels for 2030.
Summary
The levels of methane and ozone in the troposphere are closely linked via chemical reactions involving water vapour. Experiments using a coupled chemistry-climate model have shown that global methane levels are correlated with global water vapour levels, and global OH levels. Larger water vapour levels result in increased production of OH levels, and consequently smaller methane levels.
Further simulations using the coupled chemistry-climate model were used to study the effect of climate change on projected surface ozone levels for the year 2030. The water vapour levels are larger in the 2030 climate than in the 2000 climate. Climate change has reduced the projected ozone levels by 1 – 2 ppb over most of the globe. This reduction is due to the reaction between water vapour and excited state oxygen atoms. Owing to the increased water vapour levels, more excited state oxygen atoms react with water vapour, and fewer are quenched to the ground state to reform ozone. Hence, more hydroxyl radicals (OH) are made at the expense of ozone molecules, and ozone levels fall.
References
Dentener, F., Stevenson, D., Cofala, J., Mechler, R., Amann, M., Bergamaschi, P., Raes, F., and Derwent, R. 2005. The impact of air pollutant and methane emission controls on tropospheric ozone and radiative forcing: CTM calculations for the period 1990 – 2030. Atmos. Chem. Phys., 5, 1731-1755.
Dentener, F., Stevenson, D., Ellingsen, K., van Noije, T., Schultz, M., and co-authors. 2006. The global atmospheric environment for the next generation. Environ. Sci. Technol., 40, 3586-3594.
IPCC. 2000. Special Report on Emissions Scenarios: A special report of working group III of the intergovernmental panel on climate change (eds. N. Nakićenović et al.), Cambridge University Press, New York.
IPCC. 2001. Climate Change 2001: The Scientific Basis. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change (eds. J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, K. Maskell, and C. A. Johnson), Cambridge University Press, New York.
Johnson, C.E., Stevenson, D.S, Collins, W.J. and Derwent, R.G. 2001. Role of climate feedback on methane and ozone studied with a coupled Ocean-Atmosphere-Chemistry model. Geophys. Res. Lett., 28, 1723-1726.
Johnson, C.E., Stevenson, D.S., Collins, W.J., and Derwent, R.G. 2002. Interannual variability in methane growth rate simulated with a coupled ocean-atmosphere-chemistry model. Geophys. Res. Lett., 29, 1903, doi:10.1029/2002GL015269, 2002.
Stevenson, D.S., Dentener, F.J., Schultz, M.G., Ellingsen, K., Van Noije,
T.P.C., and co-authors. 2006. Multi-model ensemble simulations of present-day and near-future tropospheric ozone. J. Geophys. Res., 111, D084301, doi:10.1029/ 2005JD00638.
Vingarzan, R. 2004. A review of surface ozone background levels and trends.
Atmos. Environ., 38, 3431-3442.
Volz, A. and Kley, D. 1988. Evaluation of the Montsouris series of ozone measurements made in the nineteenth century. Nature, 332, 240-242.
Wayne, R. P. 1985. Chemistry of Atmospheres, Oxford University Press, Oxford, U.K.
West, J.J., and Fiore, A.M. 2005. Management of tropospheric ozone by reducing methane emissions. Environ. Sci. Technol., 39, 4685 -4691.
MICHAEL SANDERSON
Hadley Centre, Met Office, Fitzroy
Road, Exeter EX1 3PB
E-mail: [email protected]