The circular carbon economy and the future of carbon dioxide utilisation
Bhavin Siritanaratkul and Alexander J. Cowan
University of Liverpool
[email protected]
ECG Bulletin July 2023
University of Liverpool
[email protected]
ECG Bulletin July 2023
Carbon-containing product streams need to become more circular, embracing the use of wastes as resources. Using renewable electricity, waste CO2 can be converted into valuable fuels and chemicals in a process called electrochemical CO2 reduction. Here, the technological barriers and the outlook for the scaling-up of process are outlined.
Circular resource flows
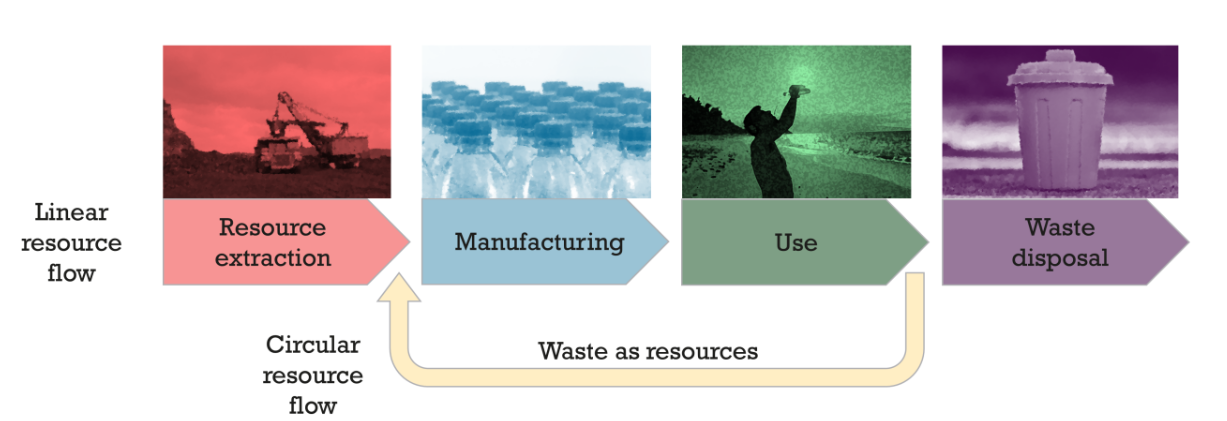
There are many definitions for circular economy, which has substantial overlap with related concepts, such as “green chemistry” and “sustainability” (1). Some processes can follow principles of green chemistry (e.g. atom economy, safe components, easily degradable waste) while still using resources in the linear fashion of “take-make-dispose” (see Figure 1). Here we use a working definition that a circular economy aims towards decreasing new resource extraction
whilst maintaining resources already in use and reducing waste generation and, in the case it cannot be reduced, the waste can be used as a resources.
There are many definitions for circular economy, which has substantial overlap with related concepts, such as “green chemistry” and “sustainability” (1). Some processes can follow principles of green chemistry (e.g. atom economy, safe components, easily degradable waste) while still using resources in the linear fashion of “take-make-dispose” (see Figure 1). Here we use a working definition that a circular economy aims towards decreasing new resource extraction
whilst maintaining resources already in use and reducing waste generation and, in the case it cannot be reduced, the waste can be used as a resources.
|
The UK has recognised the importance and urgency of implementing a circular economy and, in 2021, UK Research and Innovation (UKRI) initiated the largest investment to date in this area by creating five Research Centres, each focused on a certain resource. As part of the UKRI Interdisciplinary Centre for Circular Chemical Economy, we focus on the circular use and production of olefins (unsaturated hydrocarbons). The other Centres work on textiles, (bulk and technology) metals, and
construction materials. In addition to these resources, nitrogen and phosphorus (N- and P-) containing chemicals are also vital resource streams that need to become more circular. |
We focus on the circularity of carbon-containing resources (olefins) because:
1) Olefins represent more than 70% of industrial organic chemical production, since they are the feedstocks for manufacturing of plastics as well as rubbers, solvents, and surfactants;
2) The main source of olefins is petroleum, which is non renewable;
3) Carbon-containing waste (CO2, and to a lesser extent methane, CH4) is the main driver of global warming, and olefin production and manufacturing gives rise to 32% of total CO2 emissions from the UK chemicals sector.
1) Olefins represent more than 70% of industrial organic chemical production, since they are the feedstocks for manufacturing of plastics as well as rubbers, solvents, and surfactants;
2) The main source of olefins is petroleum, which is non renewable;
3) Carbon-containing waste (CO2, and to a lesser extent methane, CH4) is the main driver of global warming, and olefin production and manufacturing gives rise to 32% of total CO2 emissions from the UK chemicals sector.
CO2 utilisation
If we were to consider waste CO2 as a resource, it would be advantageous to use a concentrated waste stream, such as from power generation or industrial processes. Looking at CO2 emissions by sector, electricity and heat generation accounts for 42.7% of total CO2 emissions, and manufacturing and industry combines for another 21.4% (2). This suggests that majority of CO2 emissions can be potentially captured, converted, and reinserted into the resource stream, provided there is an efficient process using renewable energy as the input.
What sort of products can we aim to generate from CO2? Broadly, the products of CO2 conversion can be categorised by the number of carbon atoms as C1 and C2+ molecules. C1 products include carbon monoxide (CO), formic acid, methanol (CH3OH), and methane, all of which are important platform chemicals for industry. Carbon monoxide, together with hydrogen (also called a syngas mixture) can be further converted to higher hydrocarbons via the Fischer-Tropsch process, a collection of reactions producing liquid hydrocarbons. Methanol can be used directly as a fuel, or be further converted by the methanol-to-olefin process. C2+ molecules (such as ethylene, ethanol, acetic acid, and propanol) are intrinsically more valuable than C1 products, and they are harder to form due to the added difficulty of C–C bond formation between activated CO2 intermediates.
If we were to consider waste CO2 as a resource, it would be advantageous to use a concentrated waste stream, such as from power generation or industrial processes. Looking at CO2 emissions by sector, electricity and heat generation accounts for 42.7% of total CO2 emissions, and manufacturing and industry combines for another 21.4% (2). This suggests that majority of CO2 emissions can be potentially captured, converted, and reinserted into the resource stream, provided there is an efficient process using renewable energy as the input.
What sort of products can we aim to generate from CO2? Broadly, the products of CO2 conversion can be categorised by the number of carbon atoms as C1 and C2+ molecules. C1 products include carbon monoxide (CO), formic acid, methanol (CH3OH), and methane, all of which are important platform chemicals for industry. Carbon monoxide, together with hydrogen (also called a syngas mixture) can be further converted to higher hydrocarbons via the Fischer-Tropsch process, a collection of reactions producing liquid hydrocarbons. Methanol can be used directly as a fuel, or be further converted by the methanol-to-olefin process. C2+ molecules (such as ethylene, ethanol, acetic acid, and propanol) are intrinsically more valuable than C1 products, and they are harder to form due to the added difficulty of C–C bond formation between activated CO2 intermediates.
|
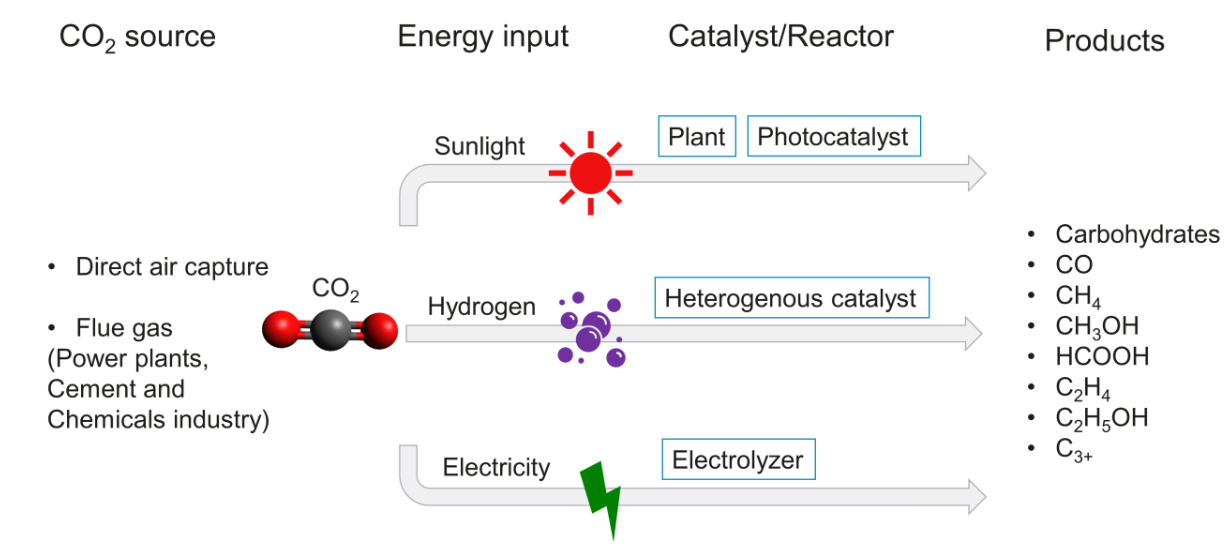
Carbon dioxide is a thermodynamically stable molecule; therefore, it needs a large energy input to convert it back to useful chemicals. The main technologies for CO2 conversion are summarised in Figure 2, categorised by the energy input. Solar energy can be used by suitable light absorbers, such as natural plants, or artificial photocatalysts and photoelectrodes. Alternatively, CO2 can be reacted with hydrogen in typically exothermic hydrogenation (3,4). This usually yields C1 products (CO, CH4, CH3OH), but bifunctional catalysts can also be used (e.g. to further convert CH3OH to longer hydrocarbons in situ).
Here, we focus on one of the most promising routes – electrochemical CO2 reduction (5). Carbon dioxide can be catalytically reduced directly with metals, molecular complexes, or enzymes, concurrently with proton transfers (most commonly from H2O). In principle, this can be a carbon-negative process, if the electricity comes from renewable sources. |
Electrochemical CO2 conversion – challenges to implementation
Lab-scale electrochemical CO2 reduction has been widely studied since pioneering work on metal electrodes in the 1980s. One key limitation which has been overcome is the CO2 supply to the electrode. In a normal batch-type cell (also called an H-cell, from the shape of two liquid compartments separated by a membrane), the limiting current density is on the scale of 12 mA/cm2. This is estimated from the CO2 solubility limit of 34 mM and a diffusion layer thickness of 100 μm, which is well below what is needed for industrial scale (> 200 mA/cm2). An electrode structure called a gas-diffusion electrode can improve CO2 transport by providing CO2 as a gas stream through a porous support layer. Recent work in the area has demonstrated devices that operate at very high current densities which are > 1 A/ cm2, similar to commercial water electrolysers. Additionally, high Faradaic efficiency has been achieved (selectivity with regard to electrons), using a variety of catalysts from metals (Cu, Ag, Sn, etc.) and metal complexes (of Mn, Ni, Co, Fe, etc.).
Lab-scale electrochemical CO2 reduction has been widely studied since pioneering work on metal electrodes in the 1980s. One key limitation which has been overcome is the CO2 supply to the electrode. In a normal batch-type cell (also called an H-cell, from the shape of two liquid compartments separated by a membrane), the limiting current density is on the scale of 12 mA/cm2. This is estimated from the CO2 solubility limit of 34 mM and a diffusion layer thickness of 100 μm, which is well below what is needed for industrial scale (> 200 mA/cm2). An electrode structure called a gas-diffusion electrode can improve CO2 transport by providing CO2 as a gas stream through a porous support layer. Recent work in the area has demonstrated devices that operate at very high current densities which are > 1 A/ cm2, similar to commercial water electrolysers. Additionally, high Faradaic efficiency has been achieved (selectivity with regard to electrons), using a variety of catalysts from metals (Cu, Ag, Sn, etc.) and metal complexes (of Mn, Ni, Co, Fe, etc.).
|
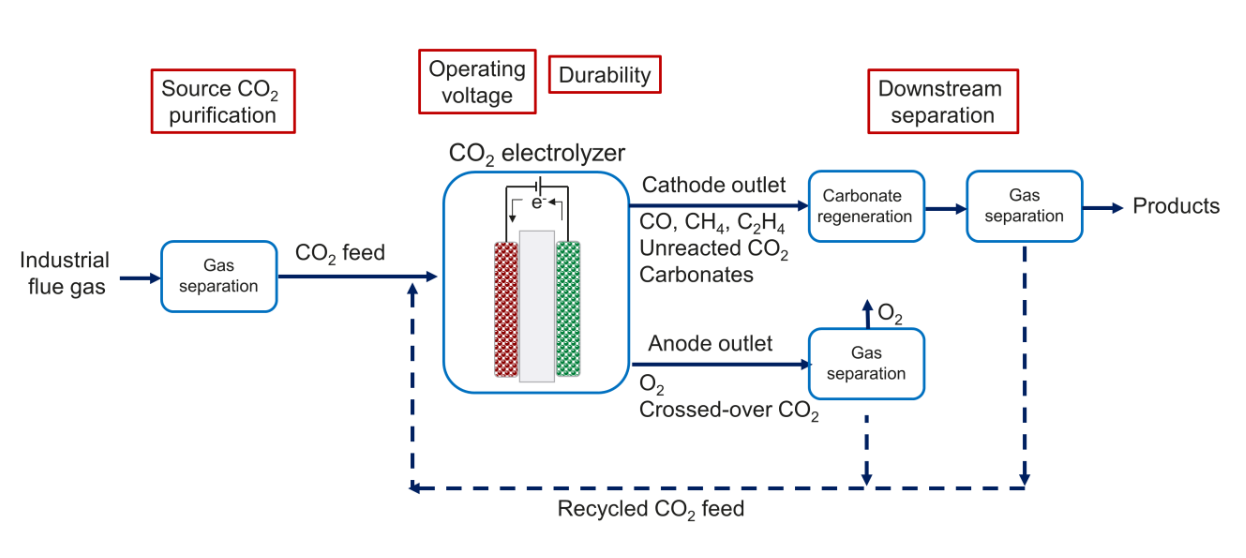
To bridge the gap between lab-scale and industrial-scale, technoeconomic analysis (TEA) has indicated that a significant part of the overall cost is from the processes upstream and downstream of the electrolyser itself (6). In Figure 3, we show a typical process flow chart of CO2 reduction, together with key parameters that we have identified as barriers to future implementation.
|
Process integration – CO2 capture and source purity
Lab-scale CO2 reduction typically uses high-purity CO2 as the feedstock, because this allows the study of the catalyst and electrolyser performance without the without the complications from contaminants. However, real-world CO2 waste streams from power generation and industrial processes (also called flue gas) will contain a mixture of other gases. Some contaminants such as H2O or N2 are benign, as they do not interfere directly with catalysis (although a large amount of diluent will lower the available CO2 amount to some extent). Other contaminants (NOx, SOx, and especially O2) are more detrimental, as they can poison the catalysts, or compete with CO2 as the reducible material, leading to most of the input electrons being wasted. Purification of input CO2, typically by amine-based capture and regeneration , adds significant cost to the overall process. One approach to circumvent this problem is to redesign the cell, for example with a perm-selective membrane, to allow less O2 to reach the catalyst and therefore remove the need for pre-purification. Another approach is to directly reduce the capture media without using energy to regenerate gaseous CO2. There are reports on direct reduction of carbonate or amine solutions, but the performances (current density and selectivity) are still below that of pure-CO2 fed systems (7). An additional problem is the degradation of the amine capture molecule, which could foul the system.
Lab-scale CO2 reduction typically uses high-purity CO2 as the feedstock, because this allows the study of the catalyst and electrolyser performance without the without the complications from contaminants. However, real-world CO2 waste streams from power generation and industrial processes (also called flue gas) will contain a mixture of other gases. Some contaminants such as H2O or N2 are benign, as they do not interfere directly with catalysis (although a large amount of diluent will lower the available CO2 amount to some extent). Other contaminants (NOx, SOx, and especially O2) are more detrimental, as they can poison the catalysts, or compete with CO2 as the reducible material, leading to most of the input electrons being wasted. Purification of input CO2, typically by amine-based capture and regeneration , adds significant cost to the overall process. One approach to circumvent this problem is to redesign the cell, for example with a perm-selective membrane, to allow less O2 to reach the catalyst and therefore remove the need for pre-purification. Another approach is to directly reduce the capture media without using energy to regenerate gaseous CO2. There are reports on direct reduction of carbonate or amine solutions, but the performances (current density and selectivity) are still below that of pure-CO2 fed systems (7). An additional problem is the degradation of the amine capture molecule, which could foul the system.
Process integration – downstream separation and regeneration
Up until the last few years, the CO2 reduction community was mainly aiming to improve the Faradaic efficiency, decreasing the proportion of electrons lost to the competing hydrogen evolution reaction. This led to the use of highly alkaline catholytes (e.g. up to 10 M KOH), since hydrogen evolution is disfavoured at low proton concentrations. However, this was in essence replacing one problem with another, as the feed CO2 reacts easily with hydroxides, forming bicarbonate and carbonate (8). This CO2 ‘lost’ as carbonates cannot directly be used by the catalyst, and they need a large energy input to regenerate CO2. An additional problem is that these undesired carbonates can cross-over through anion exchange membranes used in alkaline systems, contaminating the anode outlet stream and adding on separation costs. To circumvent this problem, we demonstrated a selective CO2 electrolyser configuration that uses bipolar membranes composed of cation and anion exchange layers (9). With the cation exchange layer towards the cathode side, this prevents carbonate generation by presenting an acidic local environment. Other cell configurations have been proposed to tackle the problem of CO2 loss (10). For example, the electrode structure and local environment can be modified to suppress hydrogen evolution and to allow operation in acidic solutions. Another approach uses a two- stage setup, where the first stage is CO2-to-CO in a solid oxide cell, and the second is CO-to-C2+ which can operate in alkaline solution since CO does not form the undesired carbonates.
|
Durability
Industrial catalytic processes typically require durability on the 10,000-hour scale, since reactor downtime and catalyst replacement would significantly increase costs. However, typical laboratory experiments are still conducted on the 10-100s of hours scale. The longest demonstration showed a device under continuous operation for 3800 hours (> 5 months) (11). |
A CO2 electrolyser is a complex device with numerous
possible failure modes. First, the catalyst can aggregate or
degrade, akin to a typical catalytic reactor. This is especially
problematic if the performance of the catalyst relies on a
certain facet (for metal catalysts) or on the dispersed
nature (of molecular complexes or single-atom materials).
Second, the ion-conducting membrane separating the
cathodes and anodes can also degrade.
Finally, the electrode structure itself is prone to flooding
and blockage, since its complex structure is necessitated by
concurrently supplying electrons, protons, and CO2 to the
catalyst. The structure of a gas-diffusion electrode is a
porous and hydrophobic conductive support, upon which a
catalyst layer is typically deposited. Ideally, the porous
support provides gas channels for fast transport of CO2 to
the catalyst, which is minimally wetted such that there is
enough H2O to provide protons, but not present a large
impediment to CO2 transport12. However, under realistic
operating conditions, H2O is generated as a by-product, as
well as transported across from the anode compartment. If
the pores in the support are flooded with excess H2O, the
CO2 supply to the catalyst will be lower, thus limiting the
achievable current. Therefore, careful water management
and monitoring is necessary.
Outlook
Electrochemical CO2 reduction is on the cusp of becoming an industrial-scale technology for converting waste CO2 back into valuable chemicals. We consider it to be at TRL (Technology Readiness Level) ~3-4 with a small number of systems reaching TRL 5, as CO2 electrolysers have been clearly demonstrated at the lab-scale, while trying to move closer to industry-relevant conditions. There are multiple start-up companies in this field, usually based on the expertise of research groups in CO2 electrochemistry. There are clear examples of electrochemical processes that have been successfully translated to a fully industrial scale. The chlor-alkali process (the electrolysis of NaCl solution to generate NaOH, H2, and Cl2) was used to generate more than 9 million tonnes of Cl2 in Europe in 2021. Water electrolysers and fuel cells have also been commercialised, and the modular nature of electrochemical cells and stacks help to simplify scaling-up once a pilot-size electrolyser is developed. With renewable electricity becoming more prevalent, electrochemical CO2 reduction can become a major pathway to achieve a circular carbon economy.
Electrochemical CO2 reduction is on the cusp of becoming an industrial-scale technology for converting waste CO2 back into valuable chemicals. We consider it to be at TRL (Technology Readiness Level) ~3-4 with a small number of systems reaching TRL 5, as CO2 electrolysers have been clearly demonstrated at the lab-scale, while trying to move closer to industry-relevant conditions. There are multiple start-up companies in this field, usually based on the expertise of research groups in CO2 electrochemistry. There are clear examples of electrochemical processes that have been successfully translated to a fully industrial scale. The chlor-alkali process (the electrolysis of NaCl solution to generate NaOH, H2, and Cl2) was used to generate more than 9 million tonnes of Cl2 in Europe in 2021. Water electrolysers and fuel cells have also been commercialised, and the modular nature of electrochemical cells and stacks help to simplify scaling-up once a pilot-size electrolyser is developed. With renewable electricity becoming more prevalent, electrochemical CO2 reduction can become a major pathway to achieve a circular carbon economy.
Acknowledgements
This work has been supported by UKRI-EPSRC funding to the Interdisciplinary Centre for the Circular Chemical Economy (EP/V011863/2).
This work has been supported by UKRI-EPSRC funding to the Interdisciplinary Centre for the Circular Chemical Economy (EP/V011863/2).
References
1. T. Keijer, V. Bakker & J.C. Slootweg, Circular chemistry to enable a circular economy. Nature Chemistry, 2019, 11 (3), 190- 195. https://doi.org/10.1038/s41557-019-0226-9
1. T. Keijer, V. Bakker & J.C. Slootweg, Circular chemistry to enable a circular economy. Nature Chemistry, 2019, 11 (3), 190- 195. https://doi.org/10.1038/s41557-019-0226-9
2. H. Ritchie, M. Roser & P. Rosado, CO2 and Greenhouse
Gas Emissions. Our World in Data, 2020.
3. A. Álvarez, A. Bansode, A. Urakawa, A.V. Bavykina, T.A. Wezendonk, M. Makkee, J. Gascon, F. Kapteijn, Challenges in the Greener Production of Formates/ Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chemical Reviews, 2017, 117 (14), 9804-9838. https://doi.org/10.1021/acs.chemrev.6b00816
4. R.-P. Ye, J. Ding, W. Gong, M.D. Argyle, Q. Zhong, Y. Wang, C.K. Russell, Z. Xu, A.G. Russell, Q. Li, M. Fan, Y.-G. Yao, CO2 hydrogenation to high-value products via heterogeneous catalysis. Nature Communications, 2019, 10 (1), 5698. https://doi.org/10.1038/s41467-019-13638-9
5. M.G. Kibria, J.P. Edwards, C.M. Gabardo, C.-T. Dinh, A. Seifitokaldani, D. Sinton, E.H. Sargent, Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Advanced Materials, 2019, 31 (31), 1807166. https://doi.org/10.1002/adma.201807166
6. A. Somoza-Tornos, O.J. Guerra, A.M. Crow, W.A. Smith, B.-M. Hodge, Process modeling, techno-economic assessment, and life cycle assessment of the electrochemical reduction of CO2: a review. iScience, 2021, 24 (7), 102813. https://doi.org/10.1016/j.isci.2021.102813
7. S.E. Jerng & B.M. Gallant, Electrochemical reduction of CO2 in the captured state using aqueous or nonaqueous amines. iScience, 2022, 25 (7), 104558. https://doi.org/10.1016/j.isci.2022.104558
3. A. Álvarez, A. Bansode, A. Urakawa, A.V. Bavykina, T.A. Wezendonk, M. Makkee, J. Gascon, F. Kapteijn, Challenges in the Greener Production of Formates/ Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chemical Reviews, 2017, 117 (14), 9804-9838. https://doi.org/10.1021/acs.chemrev.6b00816
4. R.-P. Ye, J. Ding, W. Gong, M.D. Argyle, Q. Zhong, Y. Wang, C.K. Russell, Z. Xu, A.G. Russell, Q. Li, M. Fan, Y.-G. Yao, CO2 hydrogenation to high-value products via heterogeneous catalysis. Nature Communications, 2019, 10 (1), 5698. https://doi.org/10.1038/s41467-019-13638-9
5. M.G. Kibria, J.P. Edwards, C.M. Gabardo, C.-T. Dinh, A. Seifitokaldani, D. Sinton, E.H. Sargent, Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Advanced Materials, 2019, 31 (31), 1807166. https://doi.org/10.1002/adma.201807166
6. A. Somoza-Tornos, O.J. Guerra, A.M. Crow, W.A. Smith, B.-M. Hodge, Process modeling, techno-economic assessment, and life cycle assessment of the electrochemical reduction of CO2: a review. iScience, 2021, 24 (7), 102813. https://doi.org/10.1016/j.isci.2021.102813
7. S.E. Jerng & B.M. Gallant, Electrochemical reduction of CO2 in the captured state using aqueous or nonaqueous amines. iScience, 2022, 25 (7), 104558. https://doi.org/10.1016/j.isci.2022.104558
8. J.A. Rabinowitz & M.W. Kanan, The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nature Communications, 2020, 11 (1), 5231. https://doi.org/10.1038/s41467-020-19135-8
9. B. Siritanaratkul, M. Forster, F. Greenwell, P.K. Sharma, E.H. Yu, A.J. Cowan, Zero-gap bipolar membrane electrolyzer for carbon dioxide reduction using acid-tolerant molecular electrocatalysts. Journal of the American Chemical Society, 2022, 144 (17), 7551-7556.
https://doi.org/10.1021/jacs.1c13024
10. A. Ozden, F.P. García de Arquer, J.E. Huang, J. Wicks, J. Sisler, R.K. Miao, C.P. O’Brien, G. Lee, X. Wang, A.H. Ip, E.H. Sargent, D. Sinton, Carbon-efficient carbon dioxide electrolysers. Nature Sustainability, 2022, 5 (7), 563-573. https://doi.org/10.1038/s41893-022-00879-8
9. B. Siritanaratkul, M. Forster, F. Greenwell, P.K. Sharma, E.H. Yu, A.J. Cowan, Zero-gap bipolar membrane electrolyzer for carbon dioxide reduction using acid-tolerant molecular electrocatalysts. Journal of the American Chemical Society, 2022, 144 (17), 7551-7556.
https://doi.org/10.1021/jacs.1c13024
10. A. Ozden, F.P. García de Arquer, J.E. Huang, J. Wicks, J. Sisler, R.K. Miao, C.P. O’Brien, G. Lee, X. Wang, A.H. Ip, E.H. Sargent, D. Sinton, Carbon-efficient carbon dioxide electrolysers. Nature Sustainability, 2022, 5 (7), 563-573. https://doi.org/10.1038/s41893-022-00879-8
11. Z. Liu, H. Yang, R. Kutz, & R.I. Masel, CO2 Electrolysis to
CO and O2 at high selectivity, stability and efficiency
using sustainion membranes. Journal of The
Electrochemical Society, 2018 165 (15) J3371-J3377.
https://doi.org/10.1149/2.0501815jes
12. N.T. Nesbitt, T. Burdyny, H. Simonson, D. Salvatore, D. Bohra, R. Kas, W.A. Smith, Liquid–solid boundaries dominate activity of CO2 reduction on gas-diffusion electrodes. ACS Catalysis, 2020, 10 (23), 14093-14106. https://doi.org/10.1021/acscatal.0c03319
12. N.T. Nesbitt, T. Burdyny, H. Simonson, D. Salvatore, D. Bohra, R. Kas, W.A. Smith, Liquid–solid boundaries dominate activity of CO2 reduction on gas-diffusion electrodes. ACS Catalysis, 2020, 10 (23), 14093-14106. https://doi.org/10.1021/acscatal.0c03319