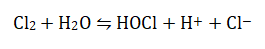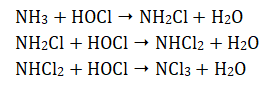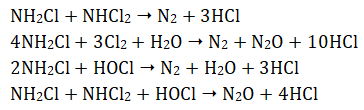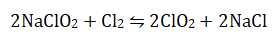Chlorine-based water disinfection
In the mid-nineteenth century, Britain suffered a succession of cholera epidemics. In 1848, there were 14,000 deaths attributable to the disease in London alone, reflecting the lack of appropriate sanitation. Famous Victorian pioneers such as Edwin Chadwick and Dr John Snow first linked the transmission of cholera to polluted drinking water. Snow demonstrated that the cholera was caused by contaminated water rather than the atmospheric 'miasma' that had previously been thought to be responsible. The memorably named Thomas Crapper carried out much of the early domestic sanitation work in the UK capital and, together with Sir Joseph Bazalgette, revolutionised the sewerage system.
Even now in the 21st century, there are still tens of thousands of deaths worldwide every day that are directly attributable to the lack of clean water. Water can be biologically contaminated with bacteria, viruses, protozoa and nematodes. Disinfecting the water enables us to destroy these disease-causing agents or pathogens, rendering the water drinkable or 'potable'.
Even now in the 21st century, there are still tens of thousands of deaths worldwide every day that are directly attributable to the lack of clean water. Water can be biologically contaminated with bacteria, viruses, protozoa and nematodes. Disinfecting the water enables us to destroy these disease-causing agents or pathogens, rendering the water drinkable or 'potable'.
Microbiology
Most outbreaks of waterborne disease are caused by contamination of water supplies with animal or human excreta. Microbiological testing is carried out to check whether water is fit for consumption by testing for the presence or concentration of a number of indicator organisms that are:
Escherichia coli is readily distinguishable and is associated with the gut of humans, mammals and fish. Whilst water containing coliform bacteria other than E.coli can be assumed to have some bacteriological contamination, water that contains E.coli is assumed to be contaminated with animal excrement. The UK Water Supply (Water Quality) Regulations detail Prescribed Concentrations and Values (PCVs), which stipulate that both E.coli. and coliform bacteria should not be detectable in any 100 ml sample volume of water.
In 1908, a British microbiologist, Dr Harriette Chick, described the reduction in microorganisms following disinfection as a first-order reaction where the rate of reduction in microorganisms with time can be expressed as:
Most outbreaks of waterborne disease are caused by contamination of water supplies with animal or human excreta. Microbiological testing is carried out to check whether water is fit for consumption by testing for the presence or concentration of a number of indicator organisms that are:
- abundant and found exclusively in faeces but (almost) absent elsewhere
- no less resistant to disinfection than other pathogens (and preferably a little more so)
- persistent in the environment
- unable to grow or multiply in the aquatic environment
- cheaply, rapidly and reliably detectable
Escherichia coli is readily distinguishable and is associated with the gut of humans, mammals and fish. Whilst water containing coliform bacteria other than E.coli can be assumed to have some bacteriological contamination, water that contains E.coli is assumed to be contaminated with animal excrement. The UK Water Supply (Water Quality) Regulations detail Prescribed Concentrations and Values (PCVs), which stipulate that both E.coli. and coliform bacteria should not be detectable in any 100 ml sample volume of water.
In 1908, a British microbiologist, Dr Harriette Chick, described the reduction in microorganisms following disinfection as a first-order reaction where the rate of reduction in microorganisms with time can be expressed as:
dN/dt = -kN
N = number of microorganisms (N0) is the initial number
k = a (disinfection) constant
t = contact time
t = contact time
The integral of this expression is known as Chick's Law and forms the basis for determining the effectiveness of a disinfection process (often simplified to depend solely on disinfectant concentration ‘c’, and the contact time 't' of the disinfectant with a pathogen):
N = N0e-kt
Disinfecting Water
A disinfectant needs to reduce pathogen concentrations within a reasonable amount of time and at a reasonable temperature. There are a number of controllable process variables, including the nature, concentration and distribution of the organisms to be destroyed, the contact time of the disinfectant with the water, the condition of the water to be treated (amount of suspended/organic matter, pH, temperature, etc.) and the presence of other compounds capable of reacting with the disinfectant. Historically throughout the UK a technique known as 'breakpoint chlorination' has been used to underpin our mains water microbiological quality.
Chlorine-based disinfection
Use of the chlorine-based disinfectant hypochlorous acid (HOCl), formed when chlorine gas mixes with water, is probably the most widely used means of drinking water disinfection in the UK. The following equilibrium is established:
A disinfectant needs to reduce pathogen concentrations within a reasonable amount of time and at a reasonable temperature. There are a number of controllable process variables, including the nature, concentration and distribution of the organisms to be destroyed, the contact time of the disinfectant with the water, the condition of the water to be treated (amount of suspended/organic matter, pH, temperature, etc.) and the presence of other compounds capable of reacting with the disinfectant. Historically throughout the UK a technique known as 'breakpoint chlorination' has been used to underpin our mains water microbiological quality.
Chlorine-based disinfection
Use of the chlorine-based disinfectant hypochlorous acid (HOCl), formed when chlorine gas mixes with water, is probably the most widely used means of drinking water disinfection in the UK. The following equilibrium is established:
Bulk chlorine gas tends to be used as the feedstock at larger water treatment sites, while pre-mixed solutions of hypochlorite salt can be used at smaller treatment works. On-site electrolytic chlorination (OSEC) is also used on some smaller sites where a concentrated solution of sodium chloride is electrolysed to form chlorine directly.
|
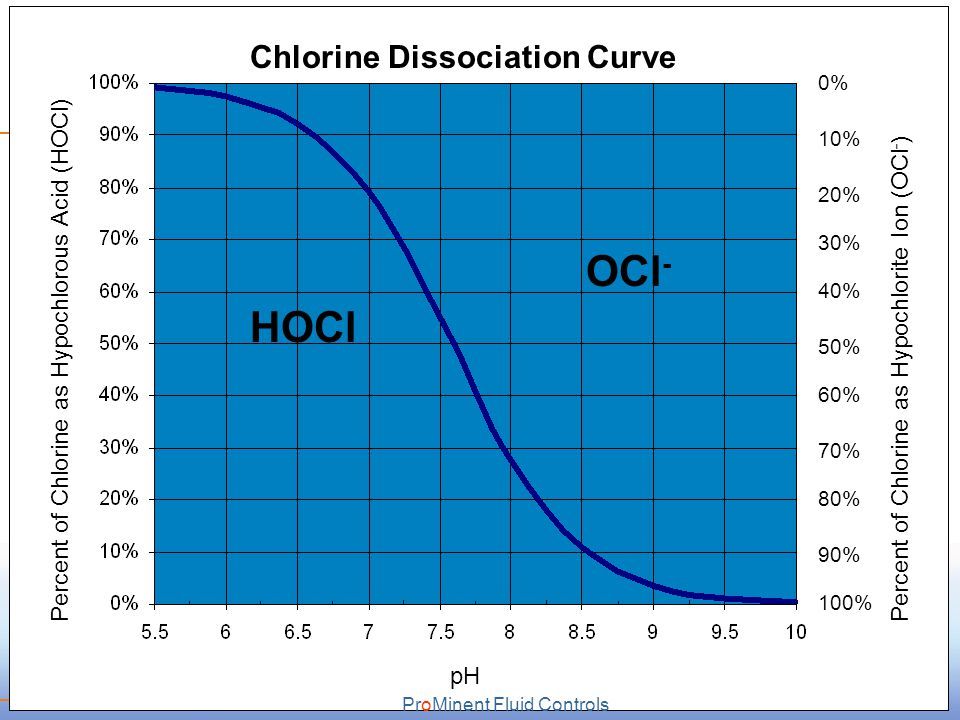
In solution, most HOCl remains undissociated below pH 7 but above pH 8, most becomes dissociated. This equilibrium is important because electronically neutral HOCl is a strong disinfectant, whereas the charged ClO− ion is 80-100 times less effective. The sum of the concentrations of HOCl and ClO− ions is known as the free available chlorine (FAC).
Ammonia can be present in natural waters and HOCl reacts with it in a stepwise manner, producing chloramines, which are weaker disinfectants.
When the molar ratios of ClO− and NH3 are similar, monochloramine (NH2Cl) tends to be the predominant reaction product.
|
The total amount of dichloramine (NHCl2) and monochloramine is called the combined available chlorine (CAC). When excess chlorine is present, more chloramines form. Eventually, all of the ammonia is removed from solution and the monochloramine and dichloramine then react together, resulting in products which are not disinfectants:
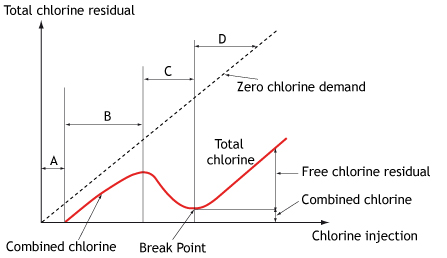
The ‘breakpoint’ occurs when the chlorine residual drops back to almost zero and all of the ammonia that was originally present has been oxidised to nitrogen. Further addition of chlorine beyond the breakpoint ensures the presence of free available chlorine and an appropriate the disinfecting environment.
|
Increasing the ratio of chlorine to ammonia beyond 5:1 results in some of the monochloramine reacting to form small amounts of dichloramine. As the ratio approaches 7.6:1 (Figure 2, C), the chloramine is oxidised by the excess chlorine to nitrogen gas, resulting in a rapid loss of residual chlorine from the water. The point between C and D (Figure 2) is the breakpoint. After this point, there is no ammonia left to react with the chlorine and the residual chlorine concentration then increases in proportion to the amount of chlorine added.
Total residual chlorine (TRC), or total chlorine, is a measure of the combined available chlorine and the free available chlorine after demand has been met.
|
It is possible that while the TRC value remains constant, the relative concentrations of the different chlorine compounds can vary widely, depending on factors such as pH and the relative concentrations of any species that can combine with chlorine.
the waterworks, freshly chlorinated water flows into a contact tank to ensure complete mixing. The effective contact period in this tank is usually defined in terms of the t10 residence time – the time for which 90% of the incoming flow has a longer residence. The target concentration for free chlorine in the water for distribution into supply is typically in the range 0.2-2.0 mg L-1 although sometimes it can be up to 5.0 mg L−1.
Sometimes a process called superchlorination is employed, involving the addition of large amounts of chlorine to destroy odours and tastes or to ensure a 'quick kill' of pathogens. If the water treated this way were then simply distributed, with its relatively high HOCl and ClO− concentrations, it would be disinfected but unpalatable. Therefore water is partially dechlorinated after superchlorination, by adding a reducing agent designed to leave a residual chlorine concentration of about 0.5 mg L−1. Usually sulphur dioxide gas (SO2) is used, although alternatives include sodium bisulfite (NaHSO3) or sodium sulfite (Na2SO3).
One of the disadvantages of using chlorine is that it can react with any natural organic matter (NOM) in the treated water to form disinfection byproducts (DBPs). The most common of these is a group of compounds called trihalomethanes (THMs). Trihalomethanes are a group of halogen-substituted organic compounds, and have the general formula CHX3 where X can be any halogen or combination of halogens and commonly include chloroform (CHCl3), bromodichloromethane (CHBrCl2), dibromochloromethane (CHBr2Cl) and bromoform (CHBr3). These THMs are all considered to be possible carcinogens, and current water quality regulations stipulate a PCV of no greater than 100 µg L−1 (total) at the tap.
Chloramination is an alternative disinfection process that involves the addition of anhydrous ammonia gas (NH3) or ammonium sulphate ((NH4)2SO4) to chlorinated water to achieve an ammonia:chlorine ratio of 1:3, and thereby intentionally generate chloramines as the disinfecting species. In the UK, this process has historically been used to disinfect much of the mains water in the London area. Chloramination offers a mechanism for long-term disinfection without creating taste problems. However, chloramination is a less powerful disinfectant than HOCl and it therefore requires a longer contact time.
Chlorine dioxide (ClO2) can also be used as a disinfectant and, although not common in the UK, is used extensively in the US. It is a gas at normal temperature and pressure and is prepared on-site from the reaction of sodium chlorite (Chloritane) with chlorine, or hydrochloric acid.
Sometimes a process called superchlorination is employed, involving the addition of large amounts of chlorine to destroy odours and tastes or to ensure a 'quick kill' of pathogens. If the water treated this way were then simply distributed, with its relatively high HOCl and ClO− concentrations, it would be disinfected but unpalatable. Therefore water is partially dechlorinated after superchlorination, by adding a reducing agent designed to leave a residual chlorine concentration of about 0.5 mg L−1. Usually sulphur dioxide gas (SO2) is used, although alternatives include sodium bisulfite (NaHSO3) or sodium sulfite (Na2SO3).
One of the disadvantages of using chlorine is that it can react with any natural organic matter (NOM) in the treated water to form disinfection byproducts (DBPs). The most common of these is a group of compounds called trihalomethanes (THMs). Trihalomethanes are a group of halogen-substituted organic compounds, and have the general formula CHX3 where X can be any halogen or combination of halogens and commonly include chloroform (CHCl3), bromodichloromethane (CHBrCl2), dibromochloromethane (CHBr2Cl) and bromoform (CHBr3). These THMs are all considered to be possible carcinogens, and current water quality regulations stipulate a PCV of no greater than 100 µg L−1 (total) at the tap.
Chloramination is an alternative disinfection process that involves the addition of anhydrous ammonia gas (NH3) or ammonium sulphate ((NH4)2SO4) to chlorinated water to achieve an ammonia:chlorine ratio of 1:3, and thereby intentionally generate chloramines as the disinfecting species. In the UK, this process has historically been used to disinfect much of the mains water in the London area. Chloramination offers a mechanism for long-term disinfection without creating taste problems. However, chloramination is a less powerful disinfectant than HOCl and it therefore requires a longer contact time.
Chlorine dioxide (ClO2) can also be used as a disinfectant and, although not common in the UK, is used extensively in the US. It is a gas at normal temperature and pressure and is prepared on-site from the reaction of sodium chlorite (Chloritane) with chlorine, or hydrochloric acid.
Normally an excess of chlorine is used to drive the reaction to the right.
Chlorine dioxide does not react with ammonia, nor create any taste problems, even at relatively high concentrations. It also has the advantage of long-lasting residual disinfection, making it particularly suitable for long distribution mains.
Chlorine dioxide does not react with ammonia, nor create any taste problems, even at relatively high concentrations. It also has the advantage of long-lasting residual disinfection, making it particularly suitable for long distribution mains.