Can mineral carbonation be used for industrial carbon dioxide sequestration?
The imperative to sequester carbon dioxide emissions from fossil fuel burning on a large scale is well recognized. Mineral carbonation is already deployed commercially and is economically viable for niche industrial outlets, but the process is not suitable, at present, for large scale applications such as power stations.
Introduction
Mineral carbonation is already deployed commercially and is economically viable for some niche industrial operations. But the technology is not yet suitable for effectively removing carbon dioxide in large scale processes such as power stations. This was the conclusion of an ESED-sponsored workshop on carbon dioxide mineralisation held on 28 November 2012 at Burlington House, London (1). However, mineral carbonation could become a significant contributor to carbon capture and storage (CCS) if:
At the moment, mineral carbonation R&D is on the edge of CCS policy, but without investment in more and focussed R&D, it cannot achieve its promise.
Carbon dioxide mineralisation is one of the cross-disciplinary cluster targets identified by the EPSRC-funded CO2Chem Network (co2chem.co.uk). The First International Conference on Accelerated Carbonation for Environmental and Materials Engineering, initiated by the UK company Carbon8 Systems Ltd, was held at the Royal Society in London in 2006; the most recent (ACEME13) was held in April 2013 in Leuven, Belgium. CO2Chem Network formed its mineralisation cluster in April 2012. The above mentioned meeting at Burlington House was this cluster’s first workshop (1). The workshop brought together about fifty active academic and industrial researchers from more than forty organisations from around the UK, plus five overseas delegates.
Introduction
Mineral carbonation is already deployed commercially and is economically viable for some niche industrial operations. But the technology is not yet suitable for effectively removing carbon dioxide in large scale processes such as power stations. This was the conclusion of an ESED-sponsored workshop on carbon dioxide mineralisation held on 28 November 2012 at Burlington House, London (1). However, mineral carbonation could become a significant contributor to carbon capture and storage (CCS) if:
- the technology can be further developed to reduce net process energy use;
- saleable carbonated products suited for low-value mega-tonne scale markets can be manufactured;
- and flue-gases can be mineralised directly.
At the moment, mineral carbonation R&D is on the edge of CCS policy, but without investment in more and focussed R&D, it cannot achieve its promise.
Carbon dioxide mineralisation is one of the cross-disciplinary cluster targets identified by the EPSRC-funded CO2Chem Network (co2chem.co.uk). The First International Conference on Accelerated Carbonation for Environmental and Materials Engineering, initiated by the UK company Carbon8 Systems Ltd, was held at the Royal Society in London in 2006; the most recent (ACEME13) was held in April 2013 in Leuven, Belgium. CO2Chem Network formed its mineralisation cluster in April 2012. The above mentioned meeting at Burlington House was this cluster’s first workshop (1). The workshop brought together about fifty active academic and industrial researchers from more than forty organisations from around the UK, plus five overseas delegates.
What is mineral carbonation?
Mineral carbonation is an approach to permanent CO2 sequestration that involves reactions of magnesium or calcium oxides (from mineral silicates or industrial wastes, rather than limestone) with CO2 to give inert carbonates. Due to the lower energy state of carbonates compared to CO2, these reactions release significant amounts of energy and, in nature, occur spontaneously (but slowly). Vast amounts of suitable and readily accessible mineral silicates exist – many times more than is needed to sequester all anthropogenic CO2 emissions.
Mineral carbonation is an approach to permanent CO2 sequestration that involves reactions of magnesium or calcium oxides (from mineral silicates or industrial wastes, rather than limestone) with CO2 to give inert carbonates. Due to the lower energy state of carbonates compared to CO2, these reactions release significant amounts of energy and, in nature, occur spontaneously (but slowly). Vast amounts of suitable and readily accessible mineral silicates exist – many times more than is needed to sequester all anthropogenic CO2 emissions.
The slow speed of reactions of natural silicate rocks (requiring mechanical, chemical or biological activation) and the large amounts of minerals or wastes that must be handled (2-3 tonnes of rock per tonne of CO2) are the main challenges for commercially viable industrial mineral carbonation applications. Processes that accelerate kinetics and maximise materials values with minimal additional costs and environmental impacts are the focus of academic research and commercial development around the world.
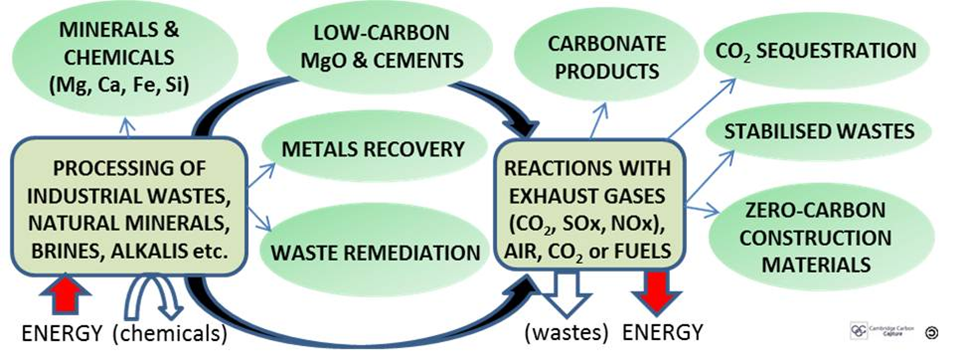
Mineral carbonation offers a commercial route to scalable CCS, starting with bottom-up, market-driven and highly profitable distributed niche applications where cost-reduction learning curves apply. Valuable and useful by-products such as silica, metals, chemicals, cements and construction materials, as well as remediation of negatively valued waste feedstocks, enable a business case to be made for CO2 sequestration even where there is no carbon price (Figure 1).
Mineral carbonation offers a commercial route to scalable CCS, starting with bottom-up, market-driven and highly profitable distributed niche applications where cost-reduction learning curves apply. Valuable and useful by-products such as silica, metals, chemicals, cements and construction materials, as well as remediation of negatively valued waste feedstocks, enable a business case to be made for CO2 sequestration even where there is no carbon price (Figure 1).
Mineral carbonation processes are increasingly being developed to sequester CO2 directly from flue gases rather than from pure CO2. This bypasses the energy-intensive and costly solvent capture/regeneration step of conventional geo-CCS and avoids the infrastructure and public acceptability challenges of supercritical CO2 pipeline transport and geological storage.
What needs to be done?
To develop mineral carbonation into a deployable engineering solution for large-scale carbon dioxide sequestration, R&D should be focussed in three main areas.
To develop mineral carbonation into a deployable engineering solution for large-scale carbon dioxide sequestration, R&D should be focussed in three main areas.
1. Energy life-cycle analysis. In principle, the reaction of Mg/Ca silicate minerals with CO2 to form carbonates and silica is exothermic and spontaneous, releasing ~0.5MWh per tonne CO2 sequestered. In practice, though, the reactions of the natural minerals are very slow (and vary significantly between them), and energy-intensive treatments, chemical additions or process conditions are applied to accelerate carbonation and recover process reactants. There are many ways to accelerate the kinetics, leading to a myriad of different mineral carbonation processes being proposed (2). Very few of these processes have been analysed to understand how much CO2 they generate for every tonne of CO2 that they sequester. The key challenge is to develop better mineral carbonation chemistries and process engineering schemes that minimise the net life-cycle energy required to sequester a tonne of CO2 as carbonate. This will include investigation of catalysts, recovery of energy released in carbonation, and recovery and recycling of process additives.
2. Scalability. At least two tonnes (and in some processes up to ten tonnes) of product materials are generated for every tonne of CO2 sequestered through carbon mineralisation. In today’s niche commercial deployments, these products—such as silica, magnesium carbonate or stabilised aggregate – are generally valuable and can be sold into available markets.
At much larger scales, for example a 0.5 GWe coal-fired power station producing ~3 million tonnes CO2 per year and 6 to 10 million tonnes per year of mineral carbonation products, more mineral feedstock than coal needs to be mined and transported to the site, and it becomes much more difficult to find sufficiently large markets into which to sell the carbonation products. In China, a 1.2 GWe coal power station is planned to be fitted with mineral carbonation technology supplied by Peabody and Calera. In this case, the carbonation products will be formatted as low-carbon construction blocks to build an adjacent new city. If mineral carbonation processes are going to be successfully applied to large scale CO2 sequestration, R&D is needed to match product materials characteristics to the technical requirements of locally available markets.
At much larger scales, for example a 0.5 GWe coal-fired power station producing ~3 million tonnes CO2 per year and 6 to 10 million tonnes per year of mineral carbonation products, more mineral feedstock than coal needs to be mined and transported to the site, and it becomes much more difficult to find sufficiently large markets into which to sell the carbonation products. In China, a 1.2 GWe coal power station is planned to be fitted with mineral carbonation technology supplied by Peabody and Calera. In this case, the carbonation products will be formatted as low-carbon construction blocks to build an adjacent new city. If mineral carbonation processes are going to be successfully applied to large scale CO2 sequestration, R&D is needed to match product materials characteristics to the technical requirements of locally available markets.
3. Dilute CO2. Post combustion capture of pure (>90%) CO2 from flue gas using regenerable solvents/sorbents is recognised at the single most costly and energy-intensive step within conventional CCS. It makes little economic or technical sense to capture CO2 first and then to mineralise it. The third area for R&D focus thus is the mineral sequestration of CO2 directly from flue gas. Yet, most research publications, funding calls and policy-level discussions in mineral carbonation are based on the premise of capture followed by mineralisation.
Mineral carbonation needs to be considered very differently from conventional geological CCS. Unlike CCS, carbonation is fundamentally an exothermic process, which is a much better starting place. It is profitable and commercially deployed at small scale, which CCS is never expected to be able to achieve. As a market-driven modular technology, it resembles wind turbines or solar power, which reduce in cost by 22% for each doubling of capacity and were once considered as unfeasibly expensive niche technologies. Like these technologies, carbon mineralisation has the potential to benefit strongly from learning curves. Given the right R&D support and market incentives, mineral carbonation has the potential to become a viable alternative to geological CCS and more than a feasible option for industrial CCS.
References
1. See http://www.rsc.org/Conferencesandevents/conference/; for speaker presentations contact [email protected].
2. M. D. Torrontégui, ‘Assessing the Mineral Carbonation Science and Technology’, Master’s Thesis, Institute of Process Engineering, ETH Zurich, Zurich, Switzerland; available online.
References
1. See http://www.rsc.org/Conferencesandevents/conference/; for speaker presentations contact [email protected].
2. M. D. Torrontégui, ‘Assessing the Mineral Carbonation Science and Technology’, Master’s Thesis, Institute of Process Engineering, ETH Zurich, Zurich, Switzerland; available online.