Biochar for soil remediation
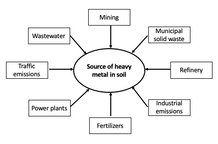
Soil contamination is a global issue predominant in developing countries where heavy metals are found in the soil above their naturally occurring concentrations (1). A major source of heavy metals occurs as a result of anthropogenic activities (Figure 1), including point source emissions from metal mining, smelting, and industrial activities and diffuse source emissions from agricultural inputs such as fertilisers, composts, sewage sludge, pesticides, and organic manures (2, 3).
|
Studies have shown that plants grown on contaminated soils tend to accumulate more heavy metals compared to plants grown on uncontaminated soil (4, 5). When consumed, these plants serve as a major pathway for heavy metals into the food chain. Adverse health problems can also result from direct ingestion or inhalation of contaminated dust (6).
|
Heavy metal contamination of soil in China is responsible for a decrease in 20 million hectares of arable land, accounting for 20% of the total agricultural land in China (7). A study carried out in Guangzhou found rice samples on the open market with Cd levels above health standards (8). The consumption of contaminated produce has been linked to accumulated heavy metals in humans, which causes adverse health issues. In Nigeria, soil contamination arises mainly from the mining and extractive industries due to the indiscriminate waste management methods. A study by the United Nations Environmental Programme has reported that Nigeria has the highest burden of pollution in Africa. For example, a United States Centre for Disease Control and Prevention study carried out in Zamfara State observed widespread lead poisoning (9). The study reported the death of over 735 children and elevated concentrations of lead in their blood. This outbreak was traced to artisanal gold mining and processing in the villages (10). Another study (11) observed elevated concentrations of lead in different vegetables grown in a farm close to a lead mine in Enyigba, Ebonyi State, Nigeria.
The persistence of heavy metals in the environment, necessitates sustainable remediation methods that can be applied to large areas of contaminated soils in low resource settings. There have been several ‘hi-tech’ approaches developed to treat and remediate contaminated soils, such as excavation, thermal treatment, bioremediation and soil vapour extraction (12, 13). However, major drawbacks to their application are their high cost and low efficiency.
What is biochar?
In recent years, biochar has received considerable attention due to its effectiveness for carbon storage, improving soil productivity, mitigating climate change, and as an adsorbent for environmental contaminants in soil and water (14-16). Biochar is a carbon-based material obtained from the pyrolysis of feedstocks under anaerobic conditions (17). The process of biochar production also serves as a waste management approach for dealing with large amounts of organic waste biomass (18). China alone produces approximately 30 million tons of sewage sludge (19, 20) and 998 million tons of dry agricultural biomass annually (18). Similarly, Nigeria produces approximately 168 million tons of agricultural waste annually (21). The use of agricultural biomass waste for biochar production shows promise as a cost-effective way to remediate contaminated soil by adsorbing and immobilising heavy metals.
The persistence of heavy metals in the environment, necessitates sustainable remediation methods that can be applied to large areas of contaminated soils in low resource settings. There have been several ‘hi-tech’ approaches developed to treat and remediate contaminated soils, such as excavation, thermal treatment, bioremediation and soil vapour extraction (12, 13). However, major drawbacks to their application are their high cost and low efficiency.
What is biochar?
In recent years, biochar has received considerable attention due to its effectiveness for carbon storage, improving soil productivity, mitigating climate change, and as an adsorbent for environmental contaminants in soil and water (14-16). Biochar is a carbon-based material obtained from the pyrolysis of feedstocks under anaerobic conditions (17). The process of biochar production also serves as a waste management approach for dealing with large amounts of organic waste biomass (18). China alone produces approximately 30 million tons of sewage sludge (19, 20) and 998 million tons of dry agricultural biomass annually (18). Similarly, Nigeria produces approximately 168 million tons of agricultural waste annually (21). The use of agricultural biomass waste for biochar production shows promise as a cost-effective way to remediate contaminated soil by adsorbing and immobilising heavy metals.
|
Farmers can prepare biochars by burning agricultural wastes in low-oxygen conditions using a modified oil barrel with holes at the bottom, which regulates the inflow of oxygen (Figure 2). The holes underneath the barrel facilitate the in-flow of primary air, and larger, L-shaped holes on the top sidewalls of the barrel facilitate the flow of secondary air. An opening is cut into the lid to support a tall chimney made from a metal pipe.
As the pyrolysis temperature increases, moisture is lost via evaporation, volatile matter is released, and decomposition of lignocellulosic material occurs, depending on feedstock type. Additionally, P, K, Ca, and Mg minerals are enriched after pyrolysis (22-24). |
Biochar as a heavy metal adsorbent
Studies on the use of biochar for soil remediation have shown that different biochars have different capacities to reduce the bioavailability of heavy metals and corresponding uptake in plants (18, 1). Effectiveness for soil remediation depends on biochar physiochemical properties, such as cation exchange capacity (CEC), surface area, elemental composition, pH, and functional groups (14). The difference between biochars in terms of adsorption of heavy metals can be linked to feedstock properties (25). The major properties of feedstocks that affect metal sorption include its C/N ratio, phosphate content, and lignin content. Biochars produced from the pyrolysis of plant-based materials will have more lignin compared to biochars produced from manure.
The C/N ratio of the biochar feedstock has been observed to influence the sorption capacity, with an increase in the sorption of Cu and Zn increasing with a decrease in the biochar feedstock C/N ratio (25). Biochar production from materials with high lignin and carbon, and with low nitrogen content favour the development of macropores on the biochar surface, which collapse as pyrolysis temperature increases, thus blocking sorption sites on its surface (15, 22). Biochar feedstocks with lower lignin content demonstrated this differently, with greater microporosity enhancing metal sorption (26). A decreased C/N ratio may be linked to the presence of low lignin content on the biochar feedstock. Conversely, greater sorption capacity has been observed from biochars produced from manure, which have high carbonate, nitrogen, and phosphate content. High sorption capacity for this these biochar types may be attributed to the mineral components, which serve as additional sorption sites (27).
Studies on the use of biochar for soil remediation have shown that different biochars have different capacities to reduce the bioavailability of heavy metals and corresponding uptake in plants (18, 1). Effectiveness for soil remediation depends on biochar physiochemical properties, such as cation exchange capacity (CEC), surface area, elemental composition, pH, and functional groups (14). The difference between biochars in terms of adsorption of heavy metals can be linked to feedstock properties (25). The major properties of feedstocks that affect metal sorption include its C/N ratio, phosphate content, and lignin content. Biochars produced from the pyrolysis of plant-based materials will have more lignin compared to biochars produced from manure.
The C/N ratio of the biochar feedstock has been observed to influence the sorption capacity, with an increase in the sorption of Cu and Zn increasing with a decrease in the biochar feedstock C/N ratio (25). Biochar production from materials with high lignin and carbon, and with low nitrogen content favour the development of macropores on the biochar surface, which collapse as pyrolysis temperature increases, thus blocking sorption sites on its surface (15, 22). Biochar feedstocks with lower lignin content demonstrated this differently, with greater microporosity enhancing metal sorption (26). A decreased C/N ratio may be linked to the presence of low lignin content on the biochar feedstock. Conversely, greater sorption capacity has been observed from biochars produced from manure, which have high carbonate, nitrogen, and phosphate content. High sorption capacity for this these biochar types may be attributed to the mineral components, which serve as additional sorption sites (27).
Biochar physiochemical properties and heavy metal adsorption
Generally, increasing pyrolysis temperature leads to an increase in the surface area of the biochar, which carries a considerable negative charge and demonstrates a strong affinity for metal cations. Thus, biochars reduce the concentration of metals in soil solutions. Increasing pyrolysis temperatures (to 300-500oC) increases the pH of the resulting biochar, due to the transformation and release of basic alkali elements like Ca2+, Mg2+, and K+ from the feedstocks (22, 24, 28). Therefore, the application of biochar to soil increases soil pH and the sorption of heavy metals to both the biochar and soil due to the deprotonation of pH-dependent cation exchange sites on soil and biochar surfaces (29). Its surface functional groups also affect metal sorption. Oxygen-containing functional groups that exist on the surface of biochars are pyrolysed at lower temperatures, but decrease in abundance with increasing pyrolysis temperature and are dependent on feedstock type. Greater adsorption at lower pyrolysis temperatures could be due to the deprotonation of oxygen-containing functional groups (carboxyl and hydroxyl), and adsorption by complexation with metal ions (30, 31).
Generally, increasing pyrolysis temperature leads to an increase in the surface area of the biochar, which carries a considerable negative charge and demonstrates a strong affinity for metal cations. Thus, biochars reduce the concentration of metals in soil solutions. Increasing pyrolysis temperatures (to 300-500oC) increases the pH of the resulting biochar, due to the transformation and release of basic alkali elements like Ca2+, Mg2+, and K+ from the feedstocks (22, 24, 28). Therefore, the application of biochar to soil increases soil pH and the sorption of heavy metals to both the biochar and soil due to the deprotonation of pH-dependent cation exchange sites on soil and biochar surfaces (29). Its surface functional groups also affect metal sorption. Oxygen-containing functional groups that exist on the surface of biochars are pyrolysed at lower temperatures, but decrease in abundance with increasing pyrolysis temperature and are dependent on feedstock type. Greater adsorption at lower pyrolysis temperatures could be due to the deprotonation of oxygen-containing functional groups (carboxyl and hydroxyl), and adsorption by complexation with metal ions (30, 31).
Application of biochar to contaminated soils
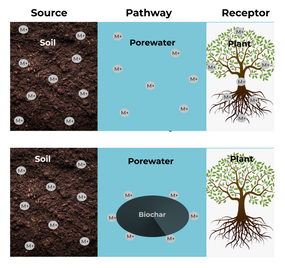
The use of biochar in heavy metal contaminated sites achieves remediation by limiting the mobility and fate of the metals in the soil. The reduction in the mobility of metals decreases their bioavailability for plant uptake. By immobilising contaminants, biochar breaks the source – pathway – receptor linkage (Figure 3), thus reducing contaminant availability to the receptor (29).
The use of biochar in heavy metal contaminated sites achieves remediation by limiting the mobility and fate of the metals in the soil. The reduction in the mobility of metals decreases their bioavailability for plant uptake. By immobilising contaminants, biochar breaks the source – pathway – receptor linkage (Figure 3), thus reducing contaminant availability to the receptor (29).
|
Several studies have demonstrated that biochar application to contaminated soils reduces and immobilises metals, eliminating the pathway to receptors (3, 13). However, one of the major drawbacks to the use of biochar for soil remediation is the presence of volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (PAHs) within the biochar itself. These compounds pose concerns and can have a major impact on organisms and the environment (32, 33). Studies have shown that the concentrations of VOCs and PAHs in biochars is dependent on the feedstock type, pyrolysis temperature, and time (34): greater concentrations of PAHs were observed with shorter pyrolysis duration and lower pyrolysis temperatures. This was attributed to the condensation of the PAHs produced on the biochar itself.
|
Research gaps
Overall, the literature to date suggests that pyrolysis temperature and feedstock type play an important role in the adsorption of heavy metals from the soil by biochar due to their impact on pH, surface area, elemental composition, and surface functional groups. However, several knowledge gaps and uncertainties remain regarding the type of feedstock, pyrolysis temperature, and application rate to optimise sorption capacity. In addition, there is a need to pay attention to the production of biochars that meet the needs of remediation without introducing organic contaminants (PAHs and VOCs) that may have deleterious effects on the environment.
References
Overall, the literature to date suggests that pyrolysis temperature and feedstock type play an important role in the adsorption of heavy metals from the soil by biochar due to their impact on pH, surface area, elemental composition, and surface functional groups. However, several knowledge gaps and uncertainties remain regarding the type of feedstock, pyrolysis temperature, and application rate to optimise sorption capacity. In addition, there is a need to pay attention to the production of biochars that meet the needs of remediation without introducing organic contaminants (PAHs and VOCs) that may have deleterious effects on the environment.
References
- Beesley, L., et al., Environmental Pollution 159, 12, 3269-3282. (2011)
- Pan, L., et al. International journal of Environmental Research and Public Health 15, 11, 2364 (2018).
- Alaboudi, K.A., Ahmed, B. and Brodie, G., Annals of Agricultural Sciences 64, 1, 95-102 (2019)
- Wang, Q-R, et al., Journal of Environmental Science and Health, Part A 38,.5, 823-838 (2003)
- Pullagurala, V.L. Reddy, et al., Science of the Total Environment 636, 1585-1596 (2018).
- Steffan, J.J., et al., European Journal of Soil Science 69, 1, 159-171,(2018)
- Zhang, X., et al., Environmental Science and Pollution Research 20, 12, 8472-8483 (2013).
- O'Connor, D., et al., Science of the Total Environment 619, 815-826 (2018)
- Tirima, S., et al., Environmental Health Perspectives, 124, 9, 1471-1478 (2016)
- Udiba, U., Akpan, E.R., and Antai, E.E., Journal of Health and Pollution, 9, 23, 190910 (2019).
- Wilberforce, Oti, J.O., and F. I. Nwabue, Environment and Pollution, 2, 1, 19 (2013)
- Lombi, E., and Hamon, R.E., Encyclopedia of Soils in the Environment Vol. 3, 379–385, Elsevier Academic Press, Amsterdam, (2005)
- Li, J., et al., Geoderma, 350, 52-60 (2019).
- Lucchini, P., et al., Agriculture, Ecosystems & Environment, 184, 149-157 (2014)
- Komnitsas, K., et al.,Waste and Biomass Valorization, 6, 5, 805-816 (2015).
- Anyanwu, I.N., et al., Data in brief, 18, 1064-1068 (2018).
- Kavitha, B., et al., Journal of Environmental Management, 227, 146-154, (2018)
- Lahori, A.H., et al., Pedosphere, 27, 6, 991-1014 (2017)
- Yu, J., Advanced Materials Research, 335-336, 1316-1320, (2011).
- Cai, L., et al., Water Research, 90, 44-51, (2016).
- Simonyan, K. J., and Fasina, O., African Journal of Agricultural Research, 8, 40, 4975-4989, (2013).
- Tan, X., et al., Chemosphere, 125, 70-85 (2015).
- Zhao, S-X., Ta, N., and Wang, X-D., Energies, 10, 9, 1293 (2017)
- Figueiredo, C., et al., Archives of Agronomy and Soil Science, 64, 6, 881-889 (2018)
- Rodríguez-Vila, A., et al., Environmental Science and Pollution Research, 25, 8, 7730-7739 (2018)
- Bogusz, A., Oleszczuk, P., and Dobrowolski, R.. Bioresource Technology, 196, 540-549 (2015)
- Xu, X., et al., Environmental Science and Pollution Research, 20, 1, 358-368 (2013)
- Zhang, R-H., et al., Ecological Engineering, 98, 183-188 (2017)
- Sizmur, T., et al. Agricultural and Environmental Applications of Biochar: Advances and Barriers, 63 295-324 (2016)
- Han, L., et al. Scientific Reports, 7, 1 1-11 (2017)
- Wang, S., et al., Royal Society Open Science, 4, 9, 170402 (2017)
- Spokas, K.A., et al., Chemosphere. 85, 5, 869-882 (2011).
- de Resende, M.F., et al., Chemosphere, 200, 641-648 (2018)
- Hale, S. E., et al. Environmental Science & Technology, 46, 5, 2830-2838 (2012)