Antibiotic resistance - antibiotic release into the environment
Antibiotics are drugs used to treat bacterial infections in humans, animals and fish. Every few weeks, major news items about the crisis of antibiotic resistance for medicinal use surface, often stating that, in the near future, there will be few antibiotics available for the treatment of infections due to overprescription. Deaths are predicted from everyday infections and safe medical procedures (1, 2). Public concern persists around the issue, and is often heightened after the publication of major national or international surveys (3-7).
The first antibiotic, penicillin, was discovered in 1928 and began to be used in 1942. Resistance was first observed in 1936. It is common for resistance to be detected within a few years of the introduction of a new antibiotic (8). No new classes of antibiotics have been brought into the market since 1987 (6).
Reports indicate resistance control must be multi-faceted, minimising levels in the wider environment (where resistance may build up) as well as controlling medicinal use in hospitals and by prescription. In some countries, antibiotic use in farming and veterinary science can exceed its use in humans, often with less strict control. Resistance built up through agricultural use may also ultimately affect human medicinal use. Legislation can be different for human and animal use and may lead to the situation where a drug strictly regulated for the former use is used more freely for the latter.
Build-up of environmental resistance
Build-up of environmental resistance depends on both chemistry and microbiology. It is first established in a bacterium in a defined geographical location. However, bacteria multiply quickly, doubling their numbers every 20-30 min. Transfer of resistance may then occur between the bacteria through the development of antibody resistance genes (ARGs), and selection pressure may favour their replication. Discussion of the role of the environment in the build up of antibiotic resistance thus runs on two parallel tracks: the build-up of resistance and the transmission of ARGs back to humans. Whether, or how, the resistance is transferred to organisms affecting human health is the subject of intense scientific debate.
The first antibiotic, penicillin, was discovered in 1928 and began to be used in 1942. Resistance was first observed in 1936. It is common for resistance to be detected within a few years of the introduction of a new antibiotic (8). No new classes of antibiotics have been brought into the market since 1987 (6).
Reports indicate resistance control must be multi-faceted, minimising levels in the wider environment (where resistance may build up) as well as controlling medicinal use in hospitals and by prescription. In some countries, antibiotic use in farming and veterinary science can exceed its use in humans, often with less strict control. Resistance built up through agricultural use may also ultimately affect human medicinal use. Legislation can be different for human and animal use and may lead to the situation where a drug strictly regulated for the former use is used more freely for the latter.
Build-up of environmental resistance
Build-up of environmental resistance depends on both chemistry and microbiology. It is first established in a bacterium in a defined geographical location. However, bacteria multiply quickly, doubling their numbers every 20-30 min. Transfer of resistance may then occur between the bacteria through the development of antibody resistance genes (ARGs), and selection pressure may favour their replication. Discussion of the role of the environment in the build up of antibiotic resistance thus runs on two parallel tracks: the build-up of resistance and the transmission of ARGs back to humans. Whether, or how, the resistance is transferred to organisms affecting human health is the subject of intense scientific debate.
|
Antibiotic types and behaviours
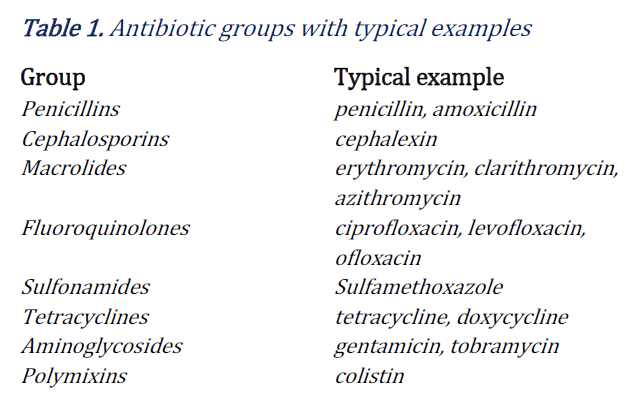
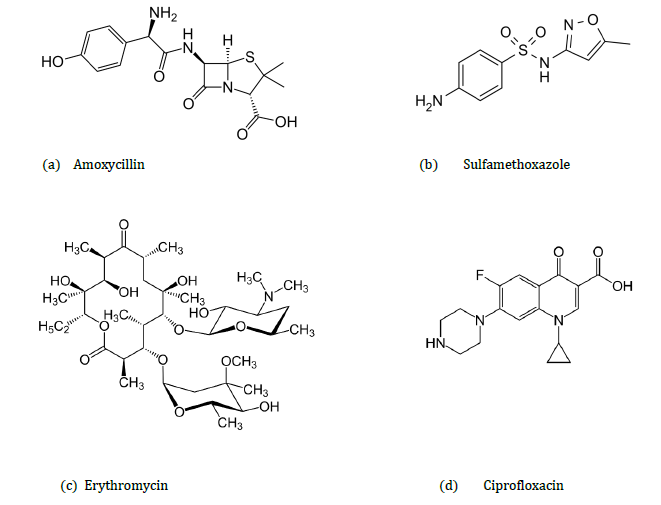
Chemical antibiotics can be divided into groups (9,10), as shown in Table 1. Antibiotics used in humans can be broad spectrum antibiotics (e.g. amoxicillin, tetracycline, aminoglycosides, and second and third generation cephalosporins), or narrow spectrum antibiotics which cause less resistance (macrolides, and older penicillins). Broad spectrum antibiotics are useful if the source of the infection is unknown. Some antibiotics, such as the fluoroquinolones and colistin, can have severe side effects and are only used in the last resort (10, 11). |
In 2013, the NHS (National Health Service, UK) reported that around one in five E. coli infections were resistant to a commonly used antibiotic (ciprofloxacin), an 18% increase since 2010. Resistance build-up is geospecific – ciprofloxacin resistance ranges from 25% in London to 12% in Cumbria, Northumberland, and Tyne and Wear (12).
Release mechanisms
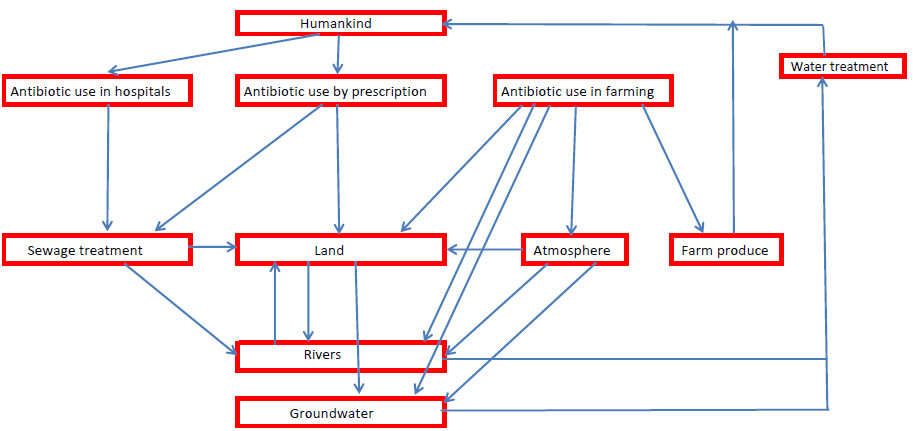
In developed countries, antibiotics primarily enter the environment through sewage treatment plants (Figure 1). Antibiotics administered medicinally pass through the body and are discharged partly as the unchanged antibiotic, partly as metabolites, and partly as glycoside or sulphate conjugates. For example, ~20% of sulfamethoxazole (a sulfonamide antibiotic) is excreted in urine unchanged, 15–20% as a glucoside conjugate, and 50–70 % as the acetylated derivative (13). Some metabolites may retain the pharmaceutical activity of the original drug. Conjugates can break down during treatment and the wider environment, reforming the original antibiotic. Unused antibiotics prescribed for home use may also be disposed of in household waste.
Release mechanisms
In developed countries, antibiotics primarily enter the environment through sewage treatment plants (Figure 1). Antibiotics administered medicinally pass through the body and are discharged partly as the unchanged antibiotic, partly as metabolites, and partly as glycoside or sulphate conjugates. For example, ~20% of sulfamethoxazole (a sulfonamide antibiotic) is excreted in urine unchanged, 15–20% as a glucoside conjugate, and 50–70 % as the acetylated derivative (13). Some metabolites may retain the pharmaceutical activity of the original drug. Conjugates can break down during treatment and the wider environment, reforming the original antibiotic. Unused antibiotics prescribed for home use may also be disposed of in household waste.
Most waste treatment plants have primary and secondary treatment processes with tertiary treatments reserved for specific contaminants which are problematic at given locations. The primary treatment is sedimentation to remove solids. Secondary treatment uses aerobic conditions to degrade organic material using populations of microorganisms. Resistance may build up in bacteria (14) during sewage digestion. Antibiotics in the sewage sludge can be returned to the environment if this digested sewage is used untreated as a soil conditioner or disposed of in landfill. The main tertiary treatment methods include ozonolysis, chlorination and UV treatment. These disinfect the water and remove specific components by chemical degradation. Removing antibiotics during sewage treatment depends not only on the treatment processes but also on the plant operational parameters such as the residence time of the wastewater (15, 16). In a study of four treatment plants (15), none of the antibiotics studied were eliminated completely, with the maximum efficiency reaching 81%. Macrolides, especially erythromycin, were stable throughout the treatment process.
|
Freshwater systems can contain a large number of pharmaceutically active compounds including antibiotics (20). Subsequent transport of antibiotics through the environment can be predicted from their chemical properties. Erythromycin (pKa 8.9, estimated KO/C 570, estimated vapour pressure 21-25 mmHg at 25°C), exists almost entirely in the cationic form in water so little volatilisation would be expected. It is expected to adsorb to suspended solids and sediment, have low mobility in soil and to exist in the atmosphere solely in the particulate phase (21).
Environmental mobility depends upon a substance’s chemical properties. Sulfamethoxazole (pKas 1.6, 5.7, estimated KO/C 72, estimated vapour pressure 6.9x10-8 mmHg at 25°C) is not expected to adsorb onto suspended solids and has low volatility in water. In general, sulfonamide antimicrobials are not readily biodegradable. They persist in soils and are highly mobile. In the atmosphere they probably exist in both vapour and particulate phases and the potential for bioconcentration in aquatic organisms is therefore estimated to be low. |
Farm animals are often given antibiotics both prophylactically and therapeutically in their water or feed. Prophylactic use is often equated to bad farming practice. Antibiotics have also been used at sub-therapeutic levels as growth hormones, but this has been banned in the EU since 2006, and, in January 2017, new rules were introduced in the US (18, 19).
Antibiotics and their metabolites can enter the environmental cycle via animal excretion, manure use (e.g. field spraying), and transfer to rivers through run-off. Farm produce is directly handled and consumed by humans. In less developed countries, where water is either not treated or poorly treated, direct consumption of water may also be a significant route.
Antibiotics and their metabolites can enter the environmental cycle via animal excretion, manure use (e.g. field spraying), and transfer to rivers through run-off. Farm produce is directly handled and consumed by humans. In less developed countries, where water is either not treated or poorly treated, direct consumption of water may also be a significant route.
Policy
Over the past three years, many major international organisations have produced extensive reports on antibiotic resistance, including campaigns to increase public awareness and improvement in sanitation, reducing the unnecessary use of antimicrobials in agriculture and their dissemination in the environment, and improving global surveillance of drug resistance and antimicrobials consumption in humans and animals.
The UK Government announced a reduction in the number of inappropriate prescriptions, working internationally to develop and make available new antibiotics, and an overall target for antibiotic use in livestock and fish farmed for food - cutting use by 2018 to the level recommended in the O’Neill report (5,6).
The US National Action Plan for combating antibiotic-resistant bacteria (4) aims to slow the emergence of resistant bacteria in both public health programmes and farming, including supervision of veterinary and agricultural uses.
Over the past three years, many major international organisations have produced extensive reports on antibiotic resistance, including campaigns to increase public awareness and improvement in sanitation, reducing the unnecessary use of antimicrobials in agriculture and their dissemination in the environment, and improving global surveillance of drug resistance and antimicrobials consumption in humans and animals.
The UK Government announced a reduction in the number of inappropriate prescriptions, working internationally to develop and make available new antibiotics, and an overall target for antibiotic use in livestock and fish farmed for food - cutting use by 2018 to the level recommended in the O’Neill report (5,6).
The US National Action Plan for combating antibiotic-resistant bacteria (4) aims to slow the emergence of resistant bacteria in both public health programmes and farming, including supervision of veterinary and agricultural uses.
|
The EU Action Plan against Antimicrobial Resistance (7) stresses the need for international cooperation and the development of new therapeutics, vaccines, and technologies.
Current EU legislation identifies the macrolide antibiotics erythromycin, clarithromycin and azithromycin as posing significant threat to environmental life, and placed them on the watch list of substances (20) as defined by Directive 2008/105/EC (21, 22). These had previously been listed by the WHO in 2011 as critically important for human health (23). Recommendations from the European Medicines Agency (EMA) for use of a human last resort antibiotic, colistin, suggest limiting its application in farming, rather than banning it completely, and improving animal husbandry rather than replacing antibiotics (24). |
The rapid publication of national and international reports is an indication that governments are realising the necessity to prevent the spread of antibiotic resistance and that control within the environment is an important tool in the overall strategy.
To learn more about this topic, register for "Biopollution: Antimicrobial Resistance in the Environment". The Distinguished Guest Lecture of the Royal Society of Chemistry Environmental Chemistry Group will be held on 28th March 2018 12:00-17:00, London, United Kingdom (see pp. 21-22 and http://www.rsc.org/events/detail/29047/biopollution-antimicrobial-resistance-in-the-environment).
To learn more about this topic, register for "Biopollution: Antimicrobial Resistance in the Environment". The Distinguished Guest Lecture of the Royal Society of Chemistry Environmental Chemistry Group will be held on 28th March 2018 12:00-17:00, London, United Kingdom (see pp. 21-22 and http://www.rsc.org/events/detail/29047/biopollution-antimicrobial-resistance-in-the-environment).
References
1. Antibiotic apocalypse: doctors sound alarm over drug resistance. www.theguardian.com/society/2017/oct/08/world-faces-antibiotic-apocalypse-says-chief-medical-officer
2. Apocalypse of antibiotic resistant superbugs will kill 10 MILLION people a year, experts fear. www.mirror.co.uk/lifestyle/health/apocalypse-antibiotic-resistant-superbugs-kill-11333481
3. Global action plan on anti-microbial resistance (WHO 2015) www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf
4. National Action Plan for combating anti-biotic resistant bacteria (US March 2015) obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
5. Tackling drug-resistant infections globally: Final report and recommendations: The review on antimicrobial resistance. Chaired by Jim O’Neill (2016) www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pd
6. Government response to the Review on Antimicrobial Resistance (September 2016) www.gov.uk/government/uploads/system/uploads/attachment_data/file/553471/Gov_response_AMR_Review.pdf
7. A European One Health Action Plan against anti-microbial resistance (June 2017) ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf
8. K. Lewis (2013) ‘Platforms for antibiotic discovery’, Nature Reviews Drug Discovery , 12, pp 371–387 doi:10.1038/nrd3975 www.nature.com/nrd/journal/v12/n5/fig_tab/nrd3975_T1.html
9. W. Yin, Hui Li, Y. Shen, Z. Liu, S. Wang, Z. Shen, R. Zhang, T.R.Walsh, J.Shen and Y.Wang (2017) ‘Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli’, 27 June mBio , 8 (3),e00543-17 mbio.asm.org/content/8/3/e00543-17
10. FDA Drug Safety Communication, www.fda.gov/Drugs/DrugSafety/ucm500143.htm
11. T. Loho T and A. Dharmayant (2015) ‘Colistin: an antibiotic and its role in multiresistant Gram-negative infections’, Acta Med Indones. 47(2) pp157-68. www.ncbi.nlm.nih.gov/pubmed/26260559
12. Antibiotic Resistance Continues to Rise, www.nhs.uk/news/medication/antibiotic-resistance-continues-to-rise/
13. McEvoy, G.K. (ed.) American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements), p. 622
14. Y. Ma, C.A. Wilson, J.T. Novak, R. Riffat, S. Aynur, S. Murphy and A. Pruden (2011) ‘Effect of Various Sludge Digestion Conditions on Sulfonamide, Macrolide and Tetracycline Resistance Genes and Class 1 Integrons’, Environmental Science and Technology, 45, pp7855-7861
15. W. Xu, G. Zhang, X. Li, Shichin Z, P. Li, Z. Hu and J. Li (2007) ‘Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD)’, South China Water Research, 41, pp 4526-4534
16. R. Reeve (2010) ‘Pharmaceuticals and sewage treatment’, Royal Society of Chemistry Environmental Chemistry Group Bulletin , January, pp 15-21, www.rsc.org/images/ECG_Jan_2010_tcm18-173535.pdf
17. V. Osorio, A. Larrañaga, J. Aceña, S. Pérez and D. Barceló (2016) ‘Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers’ , Science of The Total Environment Volume 540, 1 January 2016, Pages 267-277
18. Veterinary feed directive basics, www.avma.org/KB/Resources/Pages/VFD123.aspx
19. Ban on antibiotics as growth promoters in animal feed enters into effect, europa.eu/rapid/press-release_IP-05-1687_en.htm20. Commission implementing decision (EU) 2015/495, eur‑lex.europa.eu/legal‑content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=EN
20. Commission implementing decision (EU) 2015/495, eur‑lex.europa.eu/legal‑content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=EN
21. Environmental quality standards applicable to surface water (EU), eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:l28180&from=EN&isLegissum=true.
22. Directive amending Directives 2000/60/EC and 2008/105/EC: priority substances in water policy, eur-lex.europa.eu/legal‑content/EN/TXT/PDF/?uri=CELEX:32013L0039&from=EN
23. World Health Organisation, Important Antimicrobials for Human Medicine, (2011), apps.who.int/iris/bitstream/10665/77444/1/9789241501439_eng.pdf
24. European Medicals Agency (2016), www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/05/news_detail_002536.jsp&mid=WC0b01ac058004d5c1
1. Antibiotic apocalypse: doctors sound alarm over drug resistance. www.theguardian.com/society/2017/oct/08/world-faces-antibiotic-apocalypse-says-chief-medical-officer
2. Apocalypse of antibiotic resistant superbugs will kill 10 MILLION people a year, experts fear. www.mirror.co.uk/lifestyle/health/apocalypse-antibiotic-resistant-superbugs-kill-11333481
3. Global action plan on anti-microbial resistance (WHO 2015) www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf
4. National Action Plan for combating anti-biotic resistant bacteria (US March 2015) obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
5. Tackling drug-resistant infections globally: Final report and recommendations: The review on antimicrobial resistance. Chaired by Jim O’Neill (2016) www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pd
6. Government response to the Review on Antimicrobial Resistance (September 2016) www.gov.uk/government/uploads/system/uploads/attachment_data/file/553471/Gov_response_AMR_Review.pdf
7. A European One Health Action Plan against anti-microbial resistance (June 2017) ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf
8. K. Lewis (2013) ‘Platforms for antibiotic discovery’, Nature Reviews Drug Discovery , 12, pp 371–387 doi:10.1038/nrd3975 www.nature.com/nrd/journal/v12/n5/fig_tab/nrd3975_T1.html
9. W. Yin, Hui Li, Y. Shen, Z. Liu, S. Wang, Z. Shen, R. Zhang, T.R.Walsh, J.Shen and Y.Wang (2017) ‘Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli’, 27 June mBio , 8 (3),e00543-17 mbio.asm.org/content/8/3/e00543-17
10. FDA Drug Safety Communication, www.fda.gov/Drugs/DrugSafety/ucm500143.htm
11. T. Loho T and A. Dharmayant (2015) ‘Colistin: an antibiotic and its role in multiresistant Gram-negative infections’, Acta Med Indones. 47(2) pp157-68. www.ncbi.nlm.nih.gov/pubmed/26260559
12. Antibiotic Resistance Continues to Rise, www.nhs.uk/news/medication/antibiotic-resistance-continues-to-rise/
13. McEvoy, G.K. (ed.) American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements), p. 622
14. Y. Ma, C.A. Wilson, J.T. Novak, R. Riffat, S. Aynur, S. Murphy and A. Pruden (2011) ‘Effect of Various Sludge Digestion Conditions on Sulfonamide, Macrolide and Tetracycline Resistance Genes and Class 1 Integrons’, Environmental Science and Technology, 45, pp7855-7861
15. W. Xu, G. Zhang, X. Li, Shichin Z, P. Li, Z. Hu and J. Li (2007) ‘Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD)’, South China Water Research, 41, pp 4526-4534
16. R. Reeve (2010) ‘Pharmaceuticals and sewage treatment’, Royal Society of Chemistry Environmental Chemistry Group Bulletin , January, pp 15-21, www.rsc.org/images/ECG_Jan_2010_tcm18-173535.pdf
17. V. Osorio, A. Larrañaga, J. Aceña, S. Pérez and D. Barceló (2016) ‘Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers’ , Science of The Total Environment Volume 540, 1 January 2016, Pages 267-277
18. Veterinary feed directive basics, www.avma.org/KB/Resources/Pages/VFD123.aspx
19. Ban on antibiotics as growth promoters in animal feed enters into effect, europa.eu/rapid/press-release_IP-05-1687_en.htm20. Commission implementing decision (EU) 2015/495, eur‑lex.europa.eu/legal‑content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=EN
20. Commission implementing decision (EU) 2015/495, eur‑lex.europa.eu/legal‑content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=EN
21. Environmental quality standards applicable to surface water (EU), eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:l28180&from=EN&isLegissum=true.
22. Directive amending Directives 2000/60/EC and 2008/105/EC: priority substances in water policy, eur-lex.europa.eu/legal‑content/EN/TXT/PDF/?uri=CELEX:32013L0039&from=EN
23. World Health Organisation, Important Antimicrobials for Human Medicine, (2011), apps.who.int/iris/bitstream/10665/77444/1/9789241501439_eng.pdf
24. European Medicals Agency (2016), www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/05/news_detail_002536.jsp&mid=WC0b01ac058004d5c1