27th V. M. Goldschmidt Conference, Paris, 14th – 18th August 2017
The Goldschmidt Conference is one of the largest geochemistry conferences in the world, with more than 4,200 abstracts accepted in 2017, generating 146 different sessions, run in parallel with up to 20 different talks to choose from at any one time (goldschmidt.info/2017/history). With such a varied programme, throughout the week I was able to attend presentations on subjects as diverse as weathering and climate, isotope geochemistry, microbial metabolism and seawater chemistry, the effects of mining deep sea nodules, and even gas generation in nuclear waste repositories.
One my personal highlights of the conference was Wednesday’s plenary lecture by Hélène Langevin-Joliot of CNRS (the French National Centre for Scientific Research), talking about her life as a scientist, but also about the life and work of her rather famous grandparents, Pierre and Marie Curie. It was very interesting to hear about their personal lives, as well as their pioneering achievements in the discovery of radium and polonium.
One my personal highlights of the conference was Wednesday’s plenary lecture by Hélène Langevin-Joliot of CNRS (the French National Centre for Scientific Research), talking about her life as a scientist, but also about the life and work of her rather famous grandparents, Pierre and Marie Curie. It was very interesting to hear about their personal lives, as well as their pioneering achievements in the discovery of radium and polonium.
I chaired a session on the Friday entitled ‘The Mineralogy and Geochemistry of Metal Deposits and Secondary Sources’. We selected a range of abstracts for oral and poster presentation to encompass a range of topics relevant to the chemistry of metal deposit formation and metal recovery. The session started with talks on the geochemistry of metal deposits, covering how rare earth elements and precious metals had become enriched in ore bodies. Then the focus of the session switched to laterites, highly weathered rocks that are currently our largest source of nickel, but are increasingly being considered as a resource for cobalt and scandium (www.nhm.ac.uk/our-science/our-work/sustainability/cog3-cobalt-project.html).
The keynote talk was given by Professor Frances Wall on processing ores to recover rare earth elements (REE). There are concerns about the security of supply of REE, increasingly used in modern technologies such as wind turbines and electric cars, and therefore the European Commission has highlighted REE as critical raw materials (www.bgs.ac.uk/sosRare/home.html). It was interesting to hear about the different types of deposits containing REE. For example, mineral sands which are rich in REE and are the easiest deposit to process; however, they also contain high concentrations of uranium and thorium which means that it is unlikely that they will be exploited commercially. Absolutely crucial in the process of REE recovery is the chemistry of froth flotation which uses surfactants and frothing agents to selectively separate minerals with different hydrophobicities. This allows the REE-containing minerals to be separated from the rest of the material, and further processing is then required to separate the different REE from each other.
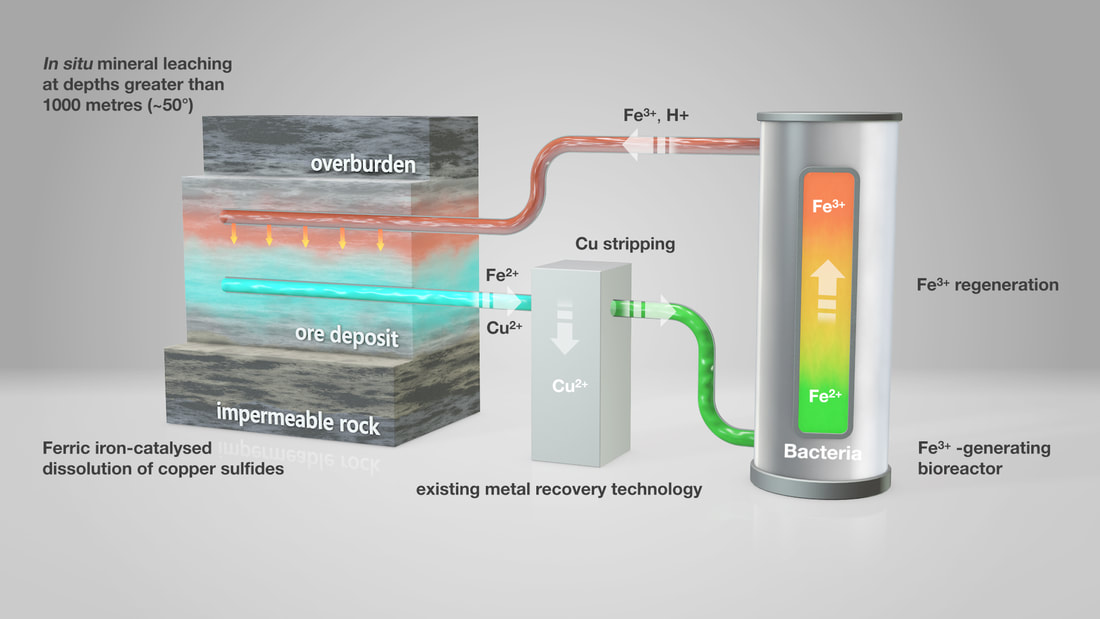
Subsequently the talks switched to research on new techniques of metal recovery, such as retrieving metals from secondary sources (e.g., mine tailings and incineration residues). These new methods are designed to be more environmentally friendly than traditional mining. One area that generated lots of discussion was the BIOMOre project (www.biomore.info). This project aims to develop innovative and environmentally sustainable technologies to recover metals from deep mineralised horizons in situ, therefore making new commodities available that are inaccessible using traditional mining techniques. The research presented involved laboratory-scale studies and modelling of bioleaching of copper sulfide minerals in the Polish Kupfershiefer sandstone deposit (Figure 1), but this is yet to be tested in the field. This technology uses iron(II)-oxidising acidophilic bacteria to produce ferric iron, which would then be pumped into the mine via horizontal fractures. The ferric iron then oxidises the sulfide minerals under acidic conditions, releasing iron(II) and copper to the aqueous phase. This leach solution is then pumped to the surface where the copper can be recovered, and the Fe(II) returned to the bioreactor for recirculation. The results presented showed that the Kupfershiefer sandstone contains carbonate minerals which will require prior removal by acid leaching as they have a significant pH buffering capacity and therefore would have an adverse impact on the bioleaching process.
The most pleasing feature of the session was an introduction to the more ‘exotic’ side of the periodic table, with many elements discussed that I had not known much about before, such as tellurium, lithium, tantalum, gallium, all of which are becoming more and more essential in the modern economy.
The keynote talk was given by Professor Frances Wall on processing ores to recover rare earth elements (REE). There are concerns about the security of supply of REE, increasingly used in modern technologies such as wind turbines and electric cars, and therefore the European Commission has highlighted REE as critical raw materials (www.bgs.ac.uk/sosRare/home.html). It was interesting to hear about the different types of deposits containing REE. For example, mineral sands which are rich in REE and are the easiest deposit to process; however, they also contain high concentrations of uranium and thorium which means that it is unlikely that they will be exploited commercially. Absolutely crucial in the process of REE recovery is the chemistry of froth flotation which uses surfactants and frothing agents to selectively separate minerals with different hydrophobicities. This allows the REE-containing minerals to be separated from the rest of the material, and further processing is then required to separate the different REE from each other.
Subsequently the talks switched to research on new techniques of metal recovery, such as retrieving metals from secondary sources (e.g., mine tailings and incineration residues). These new methods are designed to be more environmentally friendly than traditional mining. One area that generated lots of discussion was the BIOMOre project (www.biomore.info). This project aims to develop innovative and environmentally sustainable technologies to recover metals from deep mineralised horizons in situ, therefore making new commodities available that are inaccessible using traditional mining techniques. The research presented involved laboratory-scale studies and modelling of bioleaching of copper sulfide minerals in the Polish Kupfershiefer sandstone deposit (Figure 1), but this is yet to be tested in the field. This technology uses iron(II)-oxidising acidophilic bacteria to produce ferric iron, which would then be pumped into the mine via horizontal fractures. The ferric iron then oxidises the sulfide minerals under acidic conditions, releasing iron(II) and copper to the aqueous phase. This leach solution is then pumped to the surface where the copper can be recovered, and the Fe(II) returned to the bioreactor for recirculation. The results presented showed that the Kupfershiefer sandstone contains carbonate minerals which will require prior removal by acid leaching as they have a significant pH buffering capacity and therefore would have an adverse impact on the bioleaching process.
The most pleasing feature of the session was an introduction to the more ‘exotic’ side of the periodic table, with many elements discussed that I had not known much about before, such as tellurium, lithium, tantalum, gallium, all of which are becoming more and more essential in the modern economy.